dexmethylphenidate-hydrochloride
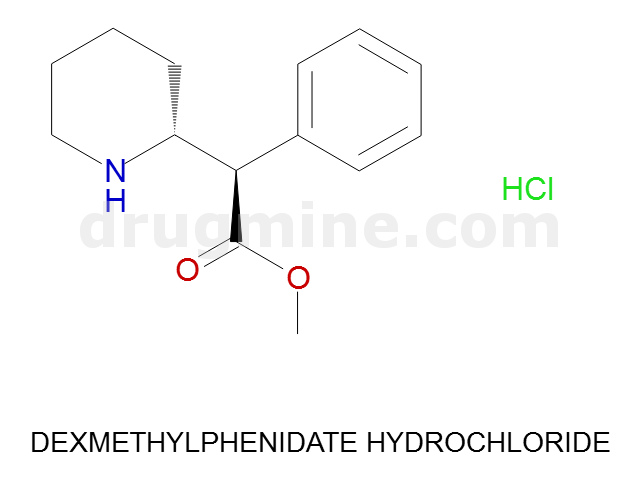

Name: DEXMETHYLPHENIDATE HYDROCHLORIDE
ID :
MW: 233
Number of atoms: 17
Molecular_Formula: C14H19NO2
Alogp: 2.18
Indication class : .
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
dexmethylphenidate hydrochloride containing products summary
There are in total 25 different products containing the active ingredient dexmethylphenidate hydrochloride. From the 25 drug products, zero have been discontinued.Product id = 21511
Application Number = 21278
Date of Application = 13,, Nov, 2001
RX/OTC/DISCN = RX
Tradename = FOCALIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 21512
Application Number = 21278
Date of Application = 13,, Nov, 2001
RX/OTC/DISCN = RX
Tradename = FOCALIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 21513
Application Number = 21278
Date of Application = 13,, Nov, 2001
RX/OTC/DISCN = RX
Tradename = FOCALIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 10MG
----
Product id = 522
Application Number = 21802
Date of Application = 26,, May, 2005
RX/OTC/DISCN = RX
Tradename = FOCALIN XR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 523
Application Number = 21802
Date of Application = 26,, May, 2005
RX/OTC/DISCN = RX
Tradename = FOCALIN XR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 525
Application Number = 21802
Date of Application = 26,, May, 2005
RX/OTC/DISCN = RX
Tradename = FOCALIN XR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 524
Application Number = 21802
Date of Application = 1,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = FOCALIN XR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 15MG
----
Product id = 527
Application Number = 21802
Date of Application = 23,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = FOCALIN XR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 005
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 529
Application Number = 21802
Date of Application = 11,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = FOCALIN XR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 006
Tecode = AB
Rld = Yes
Strength = 40MG
----
Product id = 528
Application Number = 21802
Date of Application = 21,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = FOCALIN XR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 007
Tecode =
Rld = No
Strength = 35MG
----
Product id = 526
Application Number = 21802
Date of Application = 21,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = FOCALIN XR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 008
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20258
Application Number = 77107
Date of Application = 29,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = DEXMETHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 20259
Application Number = 77107
Date of Application = 29,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = DEXMETHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 20257
Application Number = 77107
Date of Application = 29,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = DEXMETHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 395
Application Number = 78908
Date of Application = 19,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = DEXMETHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 396
Application Number = 78908
Date of Application = 19,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = DEXMETHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 398
Application Number = 78908
Date of Application = 19,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = DEXMETHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 397
Application Number = 78908
Date of Application = 19,, May, 2014
RX/OTC/DISCN = RX
Tradename = DEXMETHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 15MG
----
Product id = 392
Application Number = 78992
Date of Application = 18,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = DEXMETHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = INTELLIPHARMACEUTICS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 15MG
----
Product id = 393
Application Number = 78992
Date of Application = 18,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = DEXMETHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = INTELLIPHARMACEUTICS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 401
Application Number = 79108
Date of Application = 19,, May, 2014
RX/OTC/DISCN = RX
Tradename = DEXMETHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 15MG
----
Product id = 402
Application Number = 79108
Date of Application = 21,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = DEXMETHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 394
Application Number = 202580
Date of Application = 28,, Aug, 2013
RX/OTC/DISCN = RX
Tradename = DEXMETHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 400
Application Number = 202731
Date of Application = 19,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = DEXMETHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 399
Application Number = 202731
Date of Application = 19,, May, 2014
RX/OTC/DISCN = RX
Tradename = DEXMETHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 30MG
----