diflunisal
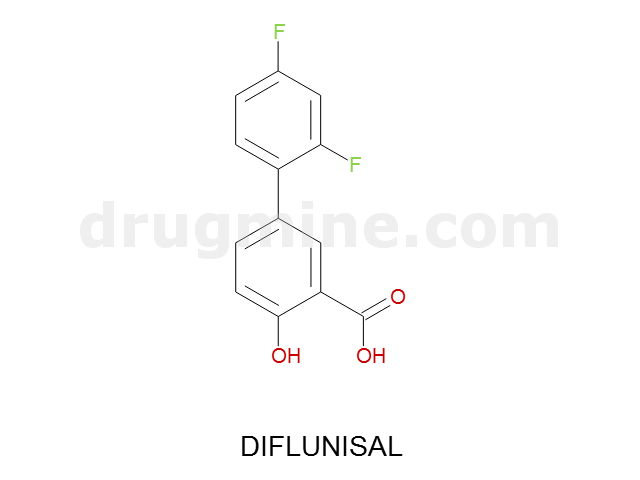
Name: DIFLUNISAL
ID :
MW: 250
Number of atoms: 18
Molecular_Formula: C13H8F2O3
Alogp: 3.147
Indication class : Analgesic; Anti-Inflammatory
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
diflunisal containing products summary
There are in total 12 different products containing the active ingredient diflunisal. From the 12 drug products, 10 have been discontinued.Product id = 20579
Application Number = 18445
Date of Application = 19,, Apr, 1982
RX/OTC/DISCN = DISCN
Tradename = DOLOBID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 20580
Application Number = 18445
Date of Application = 19,, Apr, 1982
RX/OTC/DISCN = DISCN
Tradename = DOLOBID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG
----
Product id = 20441
Application Number = 73562
Date of Application = 27,, Nov, 1992
RX/OTC/DISCN = DISCN
Tradename = DIFLUNISAL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 20442
Application Number = 73563
Date of Application = 27,, Nov, 1992
RX/OTC/DISCN = DISCN
Tradename = DIFLUNISAL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 20445
Application Number = 73673
Date of Application = 31,, Jul, 1992
RX/OTC/DISCN = RX
Tradename = DIFLUNISAL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 500MG
----
Product id = 20444
Application Number = 73679
Date of Application = 31,, Jul, 1992
RX/OTC/DISCN = DISCN
Tradename = DIFLUNISAL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 20439
Application Number = 74285
Date of Application = 7,, May, 1996
RX/OTC/DISCN = DISCN
Tradename = DIFLUNISAL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 20440
Application Number = 74285
Date of Application = 7,, May, 1996
RX/OTC/DISCN = DISCN
Tradename = DIFLUNISAL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG
----
Product id = 20446
Application Number = 74400
Date of Application = 17,, Jul, 1997
RX/OTC/DISCN = DISCN
Tradename = DIFLUNISAL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 20447
Application Number = 74400
Date of Application = 17,, Jul, 1997
RX/OTC/DISCN = DISCN
Tradename = DIFLUNISAL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG
----
Product id = 20443
Application Number = 74604
Date of Application = 10,, Jun, 1996
RX/OTC/DISCN = DISCN
Tradename = DIFLUNISAL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 20438
Application Number = 202845
Date of Application = 8,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = DIFLUNISAL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EMCURE PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----