disulfiram
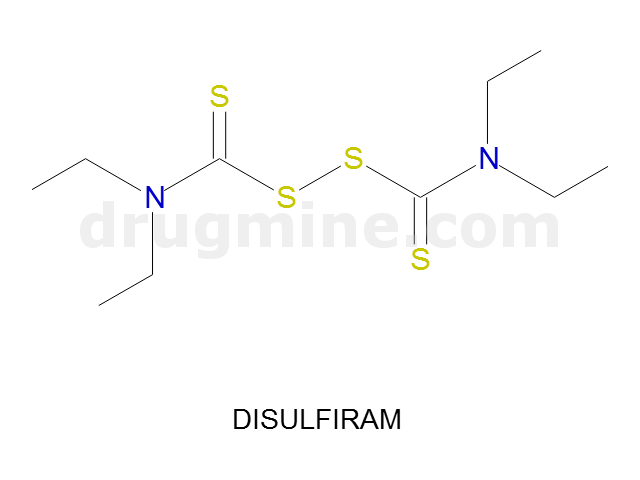

Name: DISULFIRAM
ID :
MW: 297
Number of atoms: 16
Molecular_Formula: C10H20N2S4
Alogp: 5.6
Indication class : Alcohol Deterrent
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
disulfiram containing products summary
There are in total 19 different products containing the active ingredient disulfiram. From the 19 drug products, 8 have been discontinued.Product id = 18037
Application Number = 7883
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ANTABUSE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TEVA WOMENS
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG
----
Product id = 18036
Application Number = 7883
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ANTABUSE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TEVA WOMENS
ProductNo = 003
Tecode =
Rld = No
Strength = 250MG
----
Product id = 20563
Application Number = 86889
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DISULFIRAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 20565
Application Number = 86890
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DISULFIRAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 20564
Application Number = 87973
Date of Application = 5,, Aug, 1983
RX/OTC/DISCN = DISCN
Tradename = DISULFIRAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 20566
Application Number = 87974
Date of Application = 5,, Aug, 1983
RX/OTC/DISCN = DISCN
Tradename = DISULFIRAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 18034
Application Number = 88482
Date of Application = 8,, Dec, 1983
RX/OTC/DISCN = RX
Tradename = ANTABUSE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ODYSSEY PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 18035
Application Number = 88483
Date of Application = 8,, Dec, 1983
RX/OTC/DISCN = RX
Tradename = ANTABUSE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ODYSSEY PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 500MG
----
Product id = 20555
Application Number = 88792
Date of Application = 14,, Aug, 1984
RX/OTC/DISCN = DISCN
Tradename = DISULFIRAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 20556
Application Number = 88793
Date of Application = 14,, Aug, 1984
RX/OTC/DISCN = DISCN
Tradename = DISULFIRAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 20561
Application Number = 91563
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = DISULFIRAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 20562
Application Number = 91563
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = DISULFIRAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 20559
Application Number = 91619
Date of Application = 28,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = DISULFIRAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SIGMAPHARM LABS LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 20560
Application Number = 91619
Date of Application = 28,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = DISULFIRAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SIGMAPHARM LABS LLC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 20567
Application Number = 91681
Date of Application = 8,, Aug, 2013
RX/OTC/DISCN = RX
Tradename = DISULFIRAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALVOGEN PINE BROOK
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 20557
Application Number = 202652
Date of Application = 5,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = DISULFIRAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 20558
Application Number = 202652
Date of Application = 5,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = DISULFIRAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 20553
Application Number = 203916
Date of Application = 4,, Mar, 2015
RX/OTC/DISCN = RX
Tradename = DISULFIRAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 20554
Application Number = 203916
Date of Application = 4,, Mar, 2015
RX/OTC/DISCN = RX
Tradename = DISULFIRAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG
----