dobutamine-hydrochloride
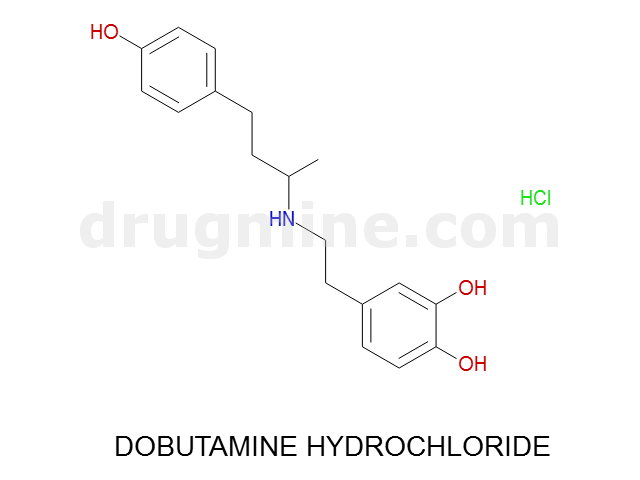


Name: DOBUTAMINE HYDROCHLORIDE
ID :
MW: 301
Number of atoms: 22
Molecular_Formula: C18H23NO3
Alogp: 3.701
Indication class : Cardiotonic
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
dobutamine hydrochloride containing products summary
There are in total 23 different products containing the active ingredient dobutamine hydrochloride. From the 23 drug products, 12 have been discontinued.Product id = 7309
Application Number = 17820
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DOBUTREX
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = LILLY
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 12.5MG BASE/ML
----
Product id = 7307
Application Number = 20201
Date of Application = 19,, Oct, 1993
RX/OTC/DISCN = RX
Tradename = DOBUTAMINE HYDROCHLORIDE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 200MG BASE/100ML
----
Product id = 7306
Application Number = 20201
Date of Application = 19,, Oct, 1993
RX/OTC/DISCN = RX
Tradename = DOBUTAMINE HYDROCHLORIDE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 100MG BASE/100ML
----
Product id = 7305
Application Number = 20201
Date of Application = 19,, Oct, 1993
RX/OTC/DISCN = RX
Tradename = DOBUTAMINE HYDROCHLORIDE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = EQ 50MG BASE/100ML
----
Product id = 7308
Application Number = 20201
Date of Application = 7,, Jul, 1994
RX/OTC/DISCN = RX
Tradename = DOBUTAMINE HYDROCHLORIDE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 006
Tecode = AP
Rld = Yes
Strength = EQ 400MG BASE/100ML
----
Product id = 7301
Application Number = 20255
Date of Application = 19,, Oct, 1993
RX/OTC/DISCN = RX
Tradename = DOBUTAMINE HYDROCHLORIDE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 50MG BASE/100ML
----
Product id = 7302
Application Number = 20255
Date of Application = 19,, Oct, 1993
RX/OTC/DISCN = RX
Tradename = DOBUTAMINE HYDROCHLORIDE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = EQ 100MG BASE/100ML
----
Product id = 7303
Application Number = 20255
Date of Application = 19,, Oct, 1993
RX/OTC/DISCN = RX
Tradename = DOBUTAMINE HYDROCHLORIDE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 004
Tecode = AP
Rld = Yes
Strength = EQ 200MG BASE/100ML
----
Product id = 7304
Application Number = 20255
Date of Application = 19,, Oct, 1993
RX/OTC/DISCN = RX
Tradename = DOBUTAMINE HYDROCHLORIDE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 005
Tecode = AP
Rld = Yes
Strength = EQ 400MG BASE/100ML
----
Product id = 7298
Application Number = 20269
Date of Application = 19,, Oct, 1993
RX/OTC/DISCN = DISCN
Tradename = DOBUTAMINE HYDROCHLORIDE IN DEXTROSE 5%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 50MG BASE/100ML
----
Product id = 7299
Application Number = 20269
Date of Application = 19,, Oct, 1993
RX/OTC/DISCN = DISCN
Tradename = DOBUTAMINE HYDROCHLORIDE IN DEXTROSE 5%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 100MG BASE/100ML
----
Product id = 7300
Application Number = 20269
Date of Application = 19,, Oct, 1993
RX/OTC/DISCN = DISCN
Tradename = DOBUTAMINE HYDROCHLORIDE IN DEXTROSE 5%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 200MG BASE/100ML
----
Product id = 7291
Application Number = 74086
Date of Application = 29,, Nov, 1993
RX/OTC/DISCN = RX
Tradename = DOBUTAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 12.5MG BASE/ML
----
Product id = 7287
Application Number = 74098
Date of Application = 21,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = DOBUTAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 12.5MG BASE/ML
----
Product id = 7295
Application Number = 74114
Date of Application = 30,, Nov, 1993
RX/OTC/DISCN = DISCN
Tradename = DOBUTAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 12.5MG BASE/ML
----
Product id = 7294
Application Number = 74206
Date of Application = 19,, Oct, 1993
RX/OTC/DISCN = DISCN
Tradename = DOBUTAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PARENTERAL
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 12.5MG BASE/ML
----
Product id = 7289
Application Number = 74277
Date of Application = 31,, Oct, 1994
RX/OTC/DISCN = RX
Tradename = DOBUTAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 12.5MG BASE/ML
----
Product id = 7296
Application Number = 74279
Date of Application = 18,, Feb, 1998
RX/OTC/DISCN = DISCN
Tradename = DOBUTAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 12.5MG BASE/ML
----
Product id = 7292
Application Number = 74292
Date of Application = 16,, Feb, 1995
RX/OTC/DISCN = RX
Tradename = DOBUTAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 12.5MG BASE/ML
----
Product id = 7288
Application Number = 74381
Date of Application = 26,, Sep, 1996
RX/OTC/DISCN = DISCN
Tradename = DOBUTAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 12.5MG BASE/ML
----
Product id = 7293
Application Number = 74545
Date of Application = 25,, Jun, 1998
RX/OTC/DISCN = DISCN
Tradename = DOBUTAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 12.5MG BASE/ML
----
Product id = 7290
Application Number = 74634
Date of Application = 27,, Sep, 1996
RX/OTC/DISCN = DISCN
Tradename = DOBUTAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1.25GM BASE/100ML
----
Product id = 7297
Application Number = 74995
Date of Application = 31,, Mar, 1998
RX/OTC/DISCN = DISCN
Tradename = DOBUTAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 12.5MG BASE/ML
----