duloxetine-hydrochloride
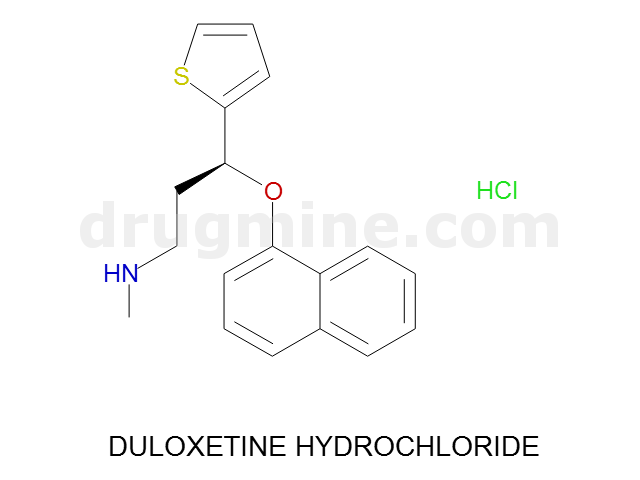


Name: DULOXETINE HYDROCHLORIDE
ID :
MW: 297
Number of atoms: 21
Molecular_Formula: C18H19NOS
Alogp: 3.846
Indication class : Antidepressant
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
duloxetine hydrochloride containing products summary
There are in total 40 different products containing the active ingredient duloxetine hydrochloride. From the 40 drug products, zero have been discontinued.Product id = 116
Application Number = 21427
Date of Application = 3,, Aug, 2004
RX/OTC/DISCN = RX
Tradename = CYMBALTA
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = N
Applicant Name = LILLY
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 117
Application Number = 21427
Date of Application = 3,, Aug, 2004
RX/OTC/DISCN = RX
Tradename = CYMBALTA
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = N
Applicant Name = LILLY
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 118
Application Number = 21427
Date of Application = 3,, Aug, 2004
RX/OTC/DISCN = RX
Tradename = CYMBALTA
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = N
Applicant Name = LILLY
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = EQ 60MG BASE
----
Product id = 152
Application Number = 90694
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 153
Application Number = 90694
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 154
Application Number = 90694
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 40MG BASE
----
Product id = 155
Application Number = 90694
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 149
Application Number = 90723
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 150
Application Number = 90723
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 151
Application Number = 90723
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 165
Application Number = 90728
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 167
Application Number = 90728
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 169
Application Number = 90728
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 166
Application Number = 90739
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 168
Application Number = 90739
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 170
Application Number = 90739
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 156
Application Number = 90745
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 157
Application Number = 90745
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 158
Application Number = 90745
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 162
Application Number = 90774
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 163
Application Number = 90774
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 164
Application Number = 90774
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 134
Application Number = 90776
Date of Application = 17,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 135
Application Number = 90776
Date of Application = 17,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 136
Application Number = 90776
Date of Application = 17,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 143
Application Number = 90778
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 144
Application Number = 90778
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 145
Application Number = 90778
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 159
Application Number = 90783
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 160
Application Number = 90783
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 161
Application Number = 90783
Date of Application = 11,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 140
Application Number = 202045
Date of Application = 11,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 141
Application Number = 202045
Date of Application = 11,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 142
Application Number = 202045
Date of Application = 11,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 137
Application Number = 202949
Date of Application = 9,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 138
Application Number = 202949
Date of Application = 9,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 139
Application Number = 202949
Date of Application = 9,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----
Product id = 146
Application Number = 203088
Date of Application = 11,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = BRECKENRIDGE PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 147
Application Number = 203088
Date of Application = 11,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = BRECKENRIDGE PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 148
Application Number = 203088
Date of Application = 11,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = DULOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = BRECKENRIDGE PHARM
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 60MG BASE
----