dutasteride
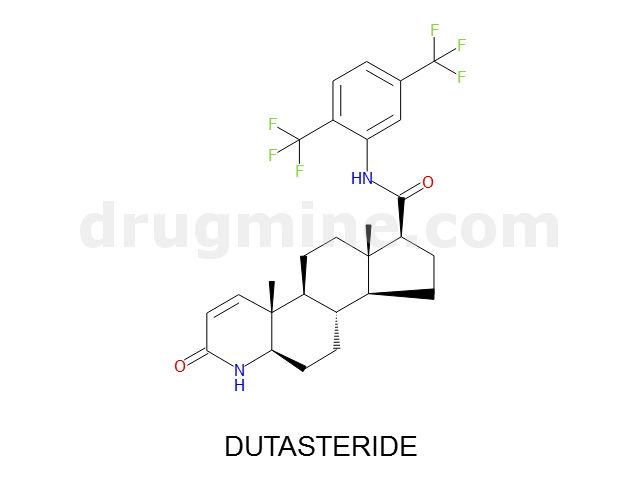

Name: DUTASTERIDE
ID :
MW: 529
Number of atoms: 37
Molecular_Formula: C27H30F6N2O2
Alogp: 5.703
Indication class : .
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
dutasteride containing products summary
There are in total 4 different products containing the active ingredient dutasteride. From the 4 drug products, zero have been discontinued.Product id = 1116
Application Number = 21319
Date of Application = 20,, Nov, 2001
RX/OTC/DISCN = RX
Tradename = AVODART
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.5MG
----
Product id = 2312
Application Number = 22460
Date of Application = 14,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = JALYN
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.5MG;0.4MG
----
Product id = 1821
Application Number = 90095
Date of Application = 21,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = DUTASTERIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 1822
Application Number = 202509
Date of Application = 26,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = DUTASTERIDE AND TAMSULOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ANCHEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG;0.4MG
----