eflornithine-hydrochloride
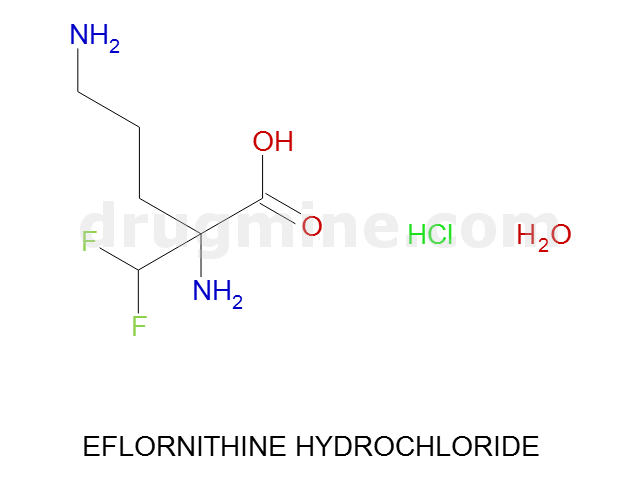


Name: EFLORNITHINE HYDROCHLORIDE
ID :
MW: 182
Number of atoms: 12
Molecular_Formula: C6H12F2N2O2
Alogp: -3.221
Indication class : Antineoplastic; Antiprotozoal
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
eflornithine hydrochloride containing products summary
There are in total 2 different products containing the active ingredient eflornithine hydrochloride. From the 2 drug products, 1 have been discontinued.Product id = 9898
Application Number = 19879
Date of Application = 28,, Nov, 1990
RX/OTC/DISCN = DISCN
Tradename = ORNIDYL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG/ML
----
Product id = 4454
Application Number = 21145
Date of Application = 27,, Jul, 2000
RX/OTC/DISCN = RX
Tradename = VANIQA
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = SKINMEDICA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 13.9%
----