fentanyl-citrate
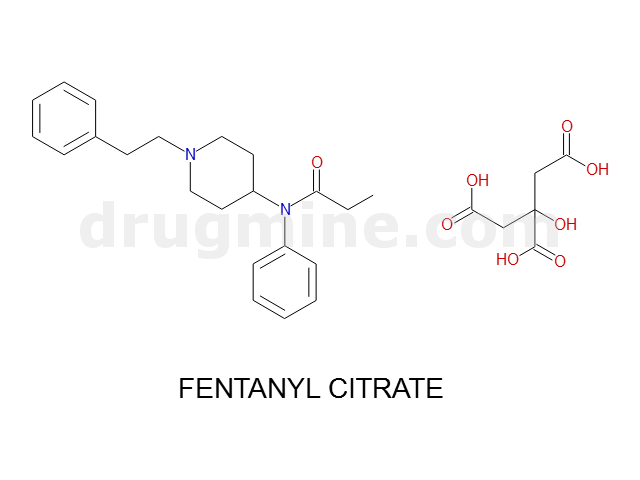
Name: FENTANYL CITRATE
ID :
MW: 336
Number of atoms: 25
Molecular_Formula: C22H28N2O
Alogp: 3.838
Indication class : Analgesic (narcotic)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
fentanyl citrate containing products summary
There are in total 59 different products containing the active ingredient fentanyl citrate. From the 59 drug products, 24 have been discontinued.Product id = 8411
Application Number = 16049
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = INNOVAR
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = AKORN MFG
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG/ML;EQ 0.05MG BASE/ML
----
Product id = 10892
Application Number = 16619
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = SUBLIMAZE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = AKORN
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 0.05MG BASE/ML
----
Product id = 7611
Application Number = 19101
Date of Application = 11,, Jul, 1984
RX/OTC/DISCN = RX
Tradename = FENTANYL CITRATE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = EUROHLTH INTL SARL
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 0.05MG BASE/ML
----
Product id = 7605
Application Number = 19115
Date of Application = 12,, Jan, 1985
RX/OTC/DISCN = RX
Tradename = FENTANYL CITRATE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 0.05MG BASE/ML
----
Product id = 30253
Application Number = 20195
Date of Application = 4,, Oct, 1993
RX/OTC/DISCN = DISCN
Tradename = FENTANYL
Route/format = ORAL / TROCHE/LOZENGE
Application Type = N
Applicant Name = CEPHALON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.2MG BASE
----
Product id = 30254
Application Number = 20195
Date of Application = 4,, Oct, 1993
RX/OTC/DISCN = DISCN
Tradename = FENTANYL
Route/format = ORAL / TROCHE/LOZENGE
Application Type = N
Applicant Name = CEPHALON
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 0.3MG BASE
----
Product id = 30255
Application Number = 20195
Date of Application = 4,, Oct, 1993
RX/OTC/DISCN = DISCN
Tradename = FENTANYL
Route/format = ORAL / TROCHE/LOZENGE
Application Type = N
Applicant Name = CEPHALON
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 0.4MG BASE
----
Product id = 30252
Application Number = 20195
Date of Application = 30,, Oct, 1995
RX/OTC/DISCN = DISCN
Tradename = FENTANYL
Route/format = ORAL / TROCHE/LOZENGE
Application Type = N
Applicant Name = CEPHALON
ProductNo = 007
Tecode =
Rld = No
Strength = EQ 0.1MG BASE
----
Product id = 30267
Application Number = 20747
Date of Application = 4,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = ACTIQ
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = N
Applicant Name = CEPHALON
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.2MG BASE
----
Product id = 30268
Application Number = 20747
Date of Application = 4,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = ACTIQ
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = N
Applicant Name = CEPHALON
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = EQ 0.4MG BASE
----
Product id = 30269
Application Number = 20747
Date of Application = 4,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = ACTIQ
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = N
Applicant Name = CEPHALON
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 0.6MG BASE
----
Product id = 30270
Application Number = 20747
Date of Application = 4,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = ACTIQ
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = N
Applicant Name = CEPHALON
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 0.8MG BASE
----
Product id = 30271
Application Number = 20747
Date of Application = 4,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = ACTIQ
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = N
Applicant Name = CEPHALON
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 1.2MG BASE
----
Product id = 30272
Application Number = 20747
Date of Application = 4,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = ACTIQ
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = N
Applicant Name = CEPHALON
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 1.6MG BASE
----
Product id = 17143
Application Number = 21947
Date of Application = 25,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = FENTORA
Route/format = BUCCAL, SUBLINGUAL / TABLET
Application Type = N
Applicant Name = CEPHALON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.1MG BASE
----
Product id = 17144
Application Number = 21947
Date of Application = 25,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = FENTORA
Route/format = BUCCAL, SUBLINGUAL / TABLET
Application Type = N
Applicant Name = CEPHALON
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 0.2MG BASE
----
Product id = 17146
Application Number = 21947
Date of Application = 25,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = FENTORA
Route/format = BUCCAL, SUBLINGUAL / TABLET
Application Type = N
Applicant Name = CEPHALON
ProductNo = 003
Tecode =
Rld = Yes
Strength = EQ 0.4MG BASE
----
Product id = 17147
Application Number = 21947
Date of Application = 25,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = FENTORA
Route/format = BUCCAL, SUBLINGUAL / TABLET
Application Type = N
Applicant Name = CEPHALON
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 0.6MG BASE
----
Product id = 17148
Application Number = 21947
Date of Application = 25,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = FENTORA
Route/format = BUCCAL, SUBLINGUAL / TABLET
Application Type = N
Applicant Name = CEPHALON
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 0.8MG BASE
----
Product id = 17145
Application Number = 21947
Date of Application = 2,, Mar, 2007
RX/OTC/DISCN = DISCN
Tradename = FENTORA
Route/format = BUCCAL, SUBLINGUAL / TABLET
Application Type = N
Applicant Name = CEPHALON
ProductNo = 006
Tecode =
Rld = No
Strength = EQ 0.3MG BASE
----
Product id = 4793
Application Number = 22266
Date of Application = 16,, Jul, 2009
RX/OTC/DISCN = DISCN
Tradename = ONSOLIS
Route/format = BUCCAL / FILM
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.2MG BASE
----
Product id = 4794
Application Number = 22266
Date of Application = 16,, Jul, 2009
RX/OTC/DISCN = DISCN
Tradename = ONSOLIS
Route/format = BUCCAL / FILM
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 0.4MG BASE
----
Product id = 4795
Application Number = 22266
Date of Application = 16,, Jul, 2009
RX/OTC/DISCN = DISCN
Tradename = ONSOLIS
Route/format = BUCCAL / FILM
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 0.6MG BASE
----
Product id = 4796
Application Number = 22266
Date of Application = 16,, Jul, 2009
RX/OTC/DISCN = DISCN
Tradename = ONSOLIS
Route/format = BUCCAL / FILM
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 0.8MG BASE
----
Product id = 4797
Application Number = 22266
Date of Application = 16,, Jul, 2009
RX/OTC/DISCN = DISCN
Tradename = ONSOLIS
Route/format = BUCCAL / FILM
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 1.2MG BASE
----
Product id = 30137
Application Number = 22510
Date of Application = 7,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = ABSTRAL
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = GALENA BIOPHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.1MG BASE
----
Product id = 30138
Application Number = 22510
Date of Application = 7,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = ABSTRAL
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = GALENA BIOPHARMA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 0.2MG BASE
----
Product id = 30139
Application Number = 22510
Date of Application = 7,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = ABSTRAL
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = GALENA BIOPHARMA
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 0.3MG BASE
----
Product id = 30140
Application Number = 22510
Date of Application = 7,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = ABSTRAL
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = GALENA BIOPHARMA
ProductNo = 004
Tecode =
Rld = Yes
Strength = EQ 0.4MG BASE
----
Product id = 30141
Application Number = 22510
Date of Application = 7,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = ABSTRAL
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = GALENA BIOPHARMA
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 0.6MG BASE
----
Product id = 30142
Application Number = 22510
Date of Application = 7,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = ABSTRAL
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = GALENA BIOPHARMA
ProductNo = 006
Tecode =
Rld = No
Strength = EQ 0.8MG BASE
----
Product id = 14377
Application Number = 22569
Date of Application = 30,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = LAZANDA
Route/format = NASAL / SPRAY, METERED
Application Type = N
Applicant Name = DEPOMED INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.1MG BASE
----
Product id = 14378
Application Number = 22569
Date of Application = 30,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = LAZANDA
Route/format = NASAL / SPRAY, METERED
Application Type = N
Applicant Name = DEPOMED INC
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 0.4MG BASE
----
Product id = 7603
Application Number = 70636
Date of Application = 30,, Apr, 1990
RX/OTC/DISCN = DISCN
Tradename = FENTANYL CITRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05MG BASE/ML
----
Product id = 7604
Application Number = 70637
Date of Application = 30,, Apr, 1990
RX/OTC/DISCN = DISCN
Tradename = FENTANYL CITRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05MG BASE/ML
----
Product id = 7610
Application Number = 71982
Date of Application = 4,, May, 1988
RX/OTC/DISCN = DISCN
Tradename = FENTANYL CITRATE AND DROPERIDOL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG/ML;EQ 0.05MG BASE/ML
----
Product id = 7607
Application Number = 72026
Date of Application = 13,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = FENTANYL CITRATE AND DROPERIDOL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG/ML;EQ 0.05MG BASE/ML
----
Product id = 7608
Application Number = 72027
Date of Application = 13,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = FENTANYL CITRATE AND DROPERIDOL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG/ML;EQ 0.05MG BASE/ML
----
Product id = 7609
Application Number = 72028
Date of Application = 13,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = FENTANYL CITRATE AND DROPERIDOL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG/ML;EQ 0.05MG BASE/ML
----
Product id = 7612
Application Number = 72786
Date of Application = 24,, Sep, 1991
RX/OTC/DISCN = RX
Tradename = FENTANYL CITRATE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 0.05MG BASE/ML
----
Product id = 7606
Application Number = 73488
Date of Application = 30,, Jun, 1992
RX/OTC/DISCN = DISCN
Tradename = FENTANYL CITRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05MG BASE/ML
----
Product id = 7613
Application Number = 74917
Date of Application = 3,, Feb, 1998
RX/OTC/DISCN = DISCN
Tradename = FENTANYL CITRATE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05MG BASE/ML
----
Product id = 30279
Application Number = 77312
Date of Application = 30,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = FENTANYL CITRATE
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.2MG BASE
----
Product id = 30280
Application Number = 77312
Date of Application = 30,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = FENTANYL CITRATE
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 0.4MG BASE
----
Product id = 30281
Application Number = 77312
Date of Application = 30,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = FENTANYL CITRATE
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 0.6MG BASE
----
Product id = 30282
Application Number = 77312
Date of Application = 30,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = FENTANYL CITRATE
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 0.8MG BASE
----
Product id = 30283
Application Number = 77312
Date of Application = 30,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = FENTANYL CITRATE
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 1.2MG BASE
----
Product id = 30284
Application Number = 77312
Date of Application = 30,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = FENTANYL CITRATE
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 1.6MG BASE
----
Product id = 30273
Application Number = 78907
Date of Application = 30,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = FENTANYL CITRATE
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.2MG BASE
----
Product id = 30274
Application Number = 78907
Date of Application = 30,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = FENTANYL CITRATE
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 0.4MG BASE
----
Product id = 30275
Application Number = 78907
Date of Application = 30,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = FENTANYL CITRATE
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 0.6MG BASE
----
Product id = 30276
Application Number = 78907
Date of Application = 30,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = FENTANYL CITRATE
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 0.8MG BASE
----
Product id = 30277
Application Number = 78907
Date of Application = 30,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = FENTANYL CITRATE
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 1.2MG BASE
----
Product id = 30278
Application Number = 78907
Date of Application = 30,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = FENTANYL CITRATE
Route/format = TRANSMUCOSAL / TROCHE/LOZENGE
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 1.6MG BASE
----
Product id = 17138
Application Number = 79075
Date of Application = 7,, Jan, 2011
RX/OTC/DISCN = DISCN
Tradename = FENTANYL CITRATE
Route/format = BUCCAL, SUBLINGUAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.1MG BASE
----
Product id = 17139
Application Number = 79075
Date of Application = 7,, Jan, 2011
RX/OTC/DISCN = DISCN
Tradename = FENTANYL CITRATE
Route/format = BUCCAL, SUBLINGUAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 0.2MG BASE
----
Product id = 17140
Application Number = 79075
Date of Application = 7,, Jan, 2011
RX/OTC/DISCN = DISCN
Tradename = FENTANYL CITRATE
Route/format = BUCCAL, SUBLINGUAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 0.4MG BASE
----
Product id = 17141
Application Number = 79075
Date of Application = 7,, Jan, 2011
RX/OTC/DISCN = DISCN
Tradename = FENTANYL CITRATE
Route/format = BUCCAL, SUBLINGUAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 0.6MG BASE
----
Product id = 17142
Application Number = 79075
Date of Application = 7,, Jan, 2011
RX/OTC/DISCN = DISCN
Tradename = FENTANYL CITRATE
Route/format = BUCCAL, SUBLINGUAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 0.8MG BASE
----