flecainide-acetate
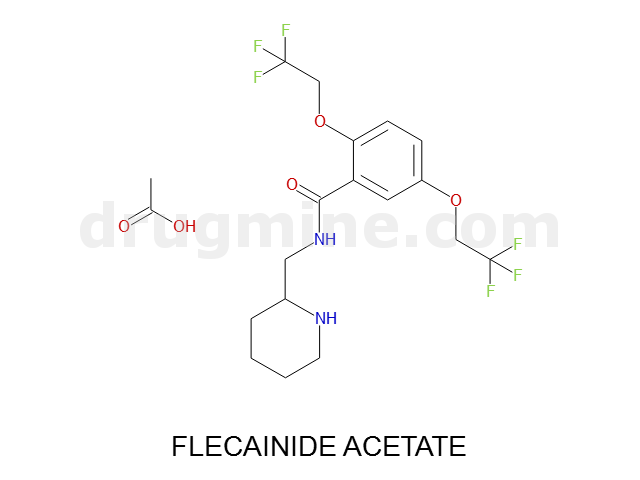
Name: FLECAINIDE ACETATE
ID :
MW: 414
Number of atoms: 28
Molecular_Formula: C17H20F6N2O3
Alogp: 3.568
Indication class : Cardiac Depressant (anti-arrhythmic)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
flecainide acetate containing products summary
There are in total 22 different products containing the active ingredient flecainide acetate. From the 22 drug products, 10 have been discontinued.Product id = 28448
Application Number = 18830
Date of Application = 31,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = TAMBOCOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = CNTY LINE PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 28450
Application Number = 18830
Date of Application = 31,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = TAMBOCOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = CNTY LINE PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG
----
Product id = 28449
Application Number = 18830
Date of Application = 3,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = TAMBOCOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = CNTY LINE PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 28447
Application Number = 18830
Date of Application = 23,, Aug, 1988
RX/OTC/DISCN = DISCN
Tradename = TAMBOCOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = CNTY LINE PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21337
Application Number = 75442
Date of Application = 31,, Jul, 2001
RX/OTC/DISCN = RX
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21338
Application Number = 75442
Date of Application = 31,, Jul, 2001
RX/OTC/DISCN = RX
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21339
Application Number = 75442
Date of Application = 31,, Jul, 2001
RX/OTC/DISCN = RX
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARM
ProductNo = 003
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 21343
Application Number = 75882
Date of Application = 28,, Oct, 2002
RX/OTC/DISCN = RX
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21344
Application Number = 75882
Date of Application = 28,, Oct, 2002
RX/OTC/DISCN = RX
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21345
Application Number = 75882
Date of Application = 28,, Oct, 2002
RX/OTC/DISCN = RX
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 003
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 21352
Application Number = 76030
Date of Application = 28,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21353
Application Number = 76030
Date of Application = 28,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21354
Application Number = 76030
Date of Application = 28,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 21349
Application Number = 76278
Date of Application = 14,, Jan, 2003
RX/OTC/DISCN = RX
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21350
Application Number = 76278
Date of Application = 14,, Jan, 2003
RX/OTC/DISCN = RX
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21351
Application Number = 76278
Date of Application = 14,, Jan, 2003
RX/OTC/DISCN = RX
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 150MG
----
Product id = 21346
Application Number = 76421
Date of Application = 28,, Mar, 2003
RX/OTC/DISCN = RX
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21347
Application Number = 76421
Date of Application = 28,, Mar, 2003
RX/OTC/DISCN = RX
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21348
Application Number = 76421
Date of Application = 28,, Mar, 2003
RX/OTC/DISCN = RX
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 21340
Application Number = 79164
Date of Application = 9,, Jul, 2009
RX/OTC/DISCN = DISCN
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21341
Application Number = 79164
Date of Application = 9,, Jul, 2009
RX/OTC/DISCN = DISCN
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21342
Application Number = 79164
Date of Application = 9,, Jul, 2009
RX/OTC/DISCN = DISCN
Tradename = FLECAINIDE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----