fluconazole
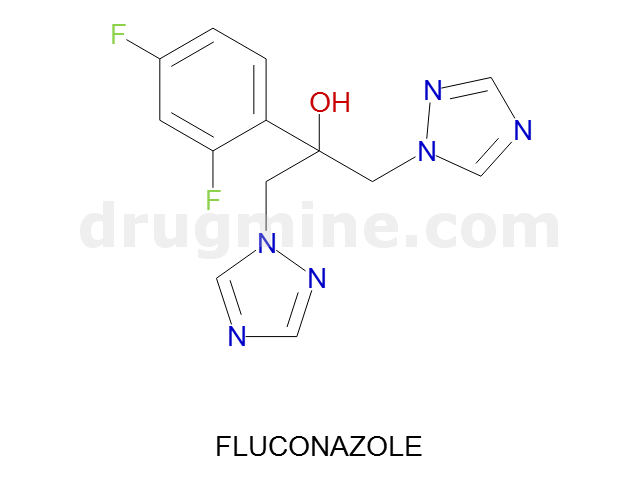

Name: FLUCONAZOLE
ID :
MW: 306
Number of atoms: 22
Molecular_Formula: C13H12F2N6O
Alogp: 0.75
Indication class : Antifungal
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
fluconazole containing products summary
There are in total 121 different products containing the active ingredient fluconazole. From the 121 drug products, 38 have been discontinued.Product id = 20434
Application Number = 19949
Date of Application = 29,, Jan, 1990
RX/OTC/DISCN = RX
Tradename = DIFLUCAN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 20435
Application Number = 19949
Date of Application = 29,, Jan, 1990
RX/OTC/DISCN = RX
Tradename = DIFLUCAN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 20437
Application Number = 19949
Date of Application = 29,, Jan, 1990
RX/OTC/DISCN = RX
Tradename = DIFLUCAN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 200MG
----
Product id = 20436
Application Number = 19949
Date of Application = 30,, Jun, 1994
RX/OTC/DISCN = RX
Tradename = DIFLUCAN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 004
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 7223
Application Number = 19950
Date of Application = 29,, Jan, 1990
RX/OTC/DISCN = RX
Tradename = DIFLUCAN IN SODIUM CHLORIDE 0.9%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7225
Application Number = 19950
Date of Application = 29,, Jan, 1990
RX/OTC/DISCN = RX
Tradename = DIFLUCAN IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7221
Application Number = 19950
Date of Application = 29,, Sep, 1992
RX/OTC/DISCN = RX
Tradename = DIFLUCAN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PFIZER
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7226
Application Number = 19950
Date of Application = 29,, Jan, 1990
RX/OTC/DISCN = RX
Tradename = DIFLUCAN IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PFIZER
ProductNo = 004
Tecode = AP
Rld = Yes
Strength = 400MG/200ML (2MG/ML)
----
Product id = 7222
Application Number = 19950
Date of Application = 8,, Jul, 1994
RX/OTC/DISCN = RX
Tradename = DIFLUCAN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PFIZER
ProductNo = 005
Tecode = AP
Rld = Yes
Strength = 400MG/200ML (2MG/ML)
----
Product id = 7224
Application Number = 19950
Date of Application = 29,, Jan, 1990
RX/OTC/DISCN = RX
Tradename = DIFLUCAN IN SODIUM CHLORIDE 0.9%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PFIZER
ProductNo = 006
Tecode = AP
Rld = Yes
Strength = 400MG/200ML (2MG/ML)
----
Product id = 5197
Application Number = 20090
Date of Application = 23,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = DIFLUCAN
Route/format = ORAL / FOR SUSPENSION
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG/5ML
----
Product id = 5198
Application Number = 20090
Date of Application = 23,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = DIFLUCAN
Route/format = ORAL / FOR SUSPENSION
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 200MG/5ML
----
Product id = 21417
Application Number = 74681
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21418
Application Number = 74681
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21419
Application Number = 74681
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 21420
Application Number = 74681
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 21393
Application Number = 76042
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21394
Application Number = 76042
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21395
Application Number = 76042
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 21396
Application Number = 76042
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG
----
Product id = 21385
Application Number = 76077
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21386
Application Number = 76077
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21387
Application Number = 76077
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 21388
Application Number = 76077
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 21409
Application Number = 76086
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21410
Application Number = 76086
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21411
Application Number = 76086
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 21412
Application Number = 76086
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG
----
Product id = 7642
Application Number = 76087
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7641
Application Number = 76087
Date of Application = 26,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG/50ML (2MG/ML)
----
Product id = 7643
Application Number = 76087
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 003
Tecode = AP
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 7646
Application Number = 76145
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7647
Application Number = 76145
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 21405
Application Number = 76213
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21406
Application Number = 76213
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21407
Application Number = 76213
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 21408
Application Number = 76213
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG
----
Product id = 5216
Application Number = 76246
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG/5ML
----
Product id = 5217
Application Number = 76246
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 200MG/5ML
----
Product id = 7661
Application Number = 76303
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7663
Application Number = 76303
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 7639
Application Number = 76304
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7640
Application Number = 76304
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 5214
Application Number = 76332
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG/5ML
----
Product id = 5215
Application Number = 76332
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 200MG/5ML
----
Product id = 21389
Application Number = 76351
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21390
Application Number = 76351
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21391
Application Number = 76351
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 21392
Application Number = 76351
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 21401
Application Number = 76386
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21402
Application Number = 76386
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21403
Application Number = 76386
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 21404
Application Number = 76386
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG
----
Product id = 21397
Application Number = 76424
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21398
Application Number = 76424
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21399
Application Number = 76424
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 21400
Application Number = 76424
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG
----
Product id = 21373
Application Number = 76432
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GEDEON RICHTER USA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21374
Application Number = 76432
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GEDEON RICHTER USA
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21375
Application Number = 76432
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GEDEON RICHTER USA
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 21376
Application Number = 76432
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GEDEON RICHTER USA
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG
----
Product id = 21413
Application Number = 76507
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21414
Application Number = 76507
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21415
Application Number = 76507
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO
ProductNo = 003
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 21416
Application Number = 76507
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO
ProductNo = 004
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 7662
Application Number = 76617
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7664
Application Number = 76617
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode =
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 7649
Application Number = 76653
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7650
Application Number = 76653
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 21369
Application Number = 76658
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21370
Application Number = 76658
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21371
Application Number = 76658
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 21372
Application Number = 76658
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 21361
Application Number = 76665
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21362
Application Number = 76665
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21363
Application Number = 76665
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 003
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 21364
Application Number = 76665
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 004
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 7648
Application Number = 76736
Date of Application = 23,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7653
Application Number = 76766
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7654
Application Number = 76766
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 7665
Application Number = 76837
Date of Application = 13,, Jan, 2005
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7666
Application Number = 76837
Date of Application = 13,, Jan, 2005
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 7633
Application Number = 76888
Date of Application = 25,, Mar, 2005
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7634
Application Number = 76888
Date of Application = 25,, Mar, 2005
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 002
Tecode =
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 7651
Application Number = 76889
Date of Application = 25,, Mar, 2005
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7652
Application Number = 76889
Date of Application = 25,, Mar, 2005
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 002
Tecode =
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 5218
Application Number = 76918
Date of Application = 18,, Dec, 2006
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = TARO PHARM INDS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/5ML
----
Product id = 5219
Application Number = 76918
Date of Application = 18,, Dec, 2006
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = TARO PHARM INDS
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG/5ML
----
Product id = 21421
Application Number = 76957
Date of Application = 28,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNIQUE PHARM LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21422
Application Number = 76957
Date of Application = 28,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNIQUE PHARM LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21423
Application Number = 76957
Date of Application = 28,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNIQUE PHARM LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 21377
Application Number = 77253
Date of Application = 25,, Jan, 2006
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21378
Application Number = 77253
Date of Application = 25,, Jan, 2006
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21379
Application Number = 77253
Date of Application = 25,, Jan, 2006
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 21380
Application Number = 77253
Date of Application = 25,, Jan, 2006
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 5212
Application Number = 77523
Date of Application = 12,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG/5ML
----
Product id = 5213
Application Number = 77523
Date of Application = 12,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 200MG/5ML
----
Product id = 21365
Application Number = 77731
Date of Application = 7,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21366
Application Number = 77731
Date of Application = 7,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21367
Application Number = 77731
Date of Application = 7,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 21368
Application Number = 77731
Date of Application = 7,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 7657
Application Number = 77909
Date of Application = 26,, May, 2010
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CLARIS LIFESCIENCES
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7658
Application Number = 77909
Date of Application = 26,, May, 2010
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CLARIS LIFESCIENCES
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 7644
Application Number = 77947
Date of Application = 26,, May, 2010
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CLARIS LIFESCIENCES
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7645
Application Number = 77947
Date of Application = 26,, May, 2010
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CLARIS LIFESCIENCES
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 7635
Application Number = 77988
Date of Application = 26,, May, 2010
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CLARIS LIFESCIENCES
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7636
Application Number = 77988
Date of Application = 26,, May, 2010
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CLARIS LIFESCIENCES
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 7655
Application Number = 78107
Date of Application = 30,, Jul, 2008
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7656
Application Number = 78107
Date of Application = 30,, Jul, 2008
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 21381
Application Number = 78423
Date of Application = 7,, Mar, 2011
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HARRIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21382
Application Number = 78423
Date of Application = 7,, Mar, 2011
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HARRIS PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21383
Application Number = 78423
Date of Application = 7,, Mar, 2011
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HARRIS PHARM
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 21384
Application Number = 78423
Date of Application = 7,, Mar, 2011
RX/OTC/DISCN = DISCN
Tradename = FLUCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HARRIS PHARM
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG
----
Product id = 7659
Application Number = 78698
Date of Application = 30,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7660
Application Number = 78698
Date of Application = 30,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 7637
Application Number = 78764
Date of Application = 30,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7638
Application Number = 78764
Date of Application = 30,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 7631
Application Number = 79104
Date of Application = 30,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = FLUCANAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR INFO SA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML (2MG/ML)
----
Product id = 7632
Application Number = 79104
Date of Application = 30,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = FLUCANAZOLE IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR INFO SA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/200ML (2MG/ML)
----
Product id = 5210
Application Number = 79150
Date of Application = 18,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = AUROBINDO PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG/5ML
----
Product id = 5211
Application Number = 79150
Date of Application = 18,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = FLUCONAZOLE
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = AUROBINDO PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 200MG/5ML
----