fluoxetine-hydrochloride
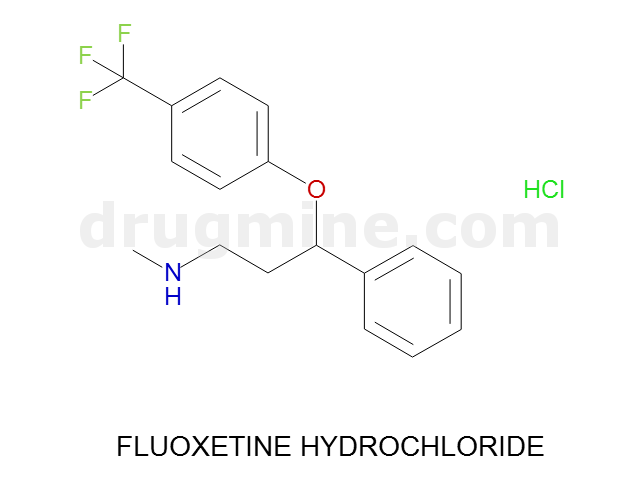


Name: FLUOXETINE HYDROCHLORIDE
ID :
MW: 309
Number of atoms: 22
Molecular_Formula: C17H18F3NO
Alogp: 4.033
Indication class : Antidepressant
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
fluoxetine hydrochloride containing products summary
There are in total 115 different products containing the active ingredient fluoxetine hydrochloride. From the 115 drug products, 27 have been discontinued.Product id = 3083
Application Number = 18936
Date of Application = 29,, Dec, 1987
RX/OTC/DISCN = RX
Tradename = PROZAC
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ELI LILLY AND CO
ProductNo = 001
Tecode = AB1
Rld = No
Strength = EQ 20MG BASE
----
Product id = 3084
Application Number = 18936
Date of Application = 15,, Jun, 1999
RX/OTC/DISCN = RX
Tradename = PROZAC
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ELI LILLY AND CO
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = EQ 40MG BASE
----
Product id = 3085
Application Number = 18936
Date of Application = 15,, Jun, 1999
RX/OTC/DISCN = DISCN
Tradename = PROZAC
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ELI LILLY AND CO
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 60MG BASE
----
Product id = 3082
Application Number = 18936
Date of Application = 23,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = PROZAC
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ELI LILLY AND CO
ProductNo = 006
Tecode = AB1
Rld = No
Strength = EQ 10MG BASE
----
Product id = 3236
Application Number = 18936
Date of Application = 6,, Jul, 2000
RX/OTC/DISCN = RX
Tradename = SARAFEM
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ELI LILLY AND CO
ProductNo = 007
Tecode = AB2
Rld = No
Strength = EQ 10MG BASE
----
Product id = 3237
Application Number = 18936
Date of Application = 6,, Jul, 2000
RX/OTC/DISCN = RX
Tradename = SARAFEM
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ELI LILLY AND CO
ProductNo = 008
Tecode = AB2
Rld = Yes
Strength = EQ 20MG BASE
----
Product id = 14014
Application Number = 20101
Date of Application = 24,, Apr, 1991
RX/OTC/DISCN = DISCN
Tradename = PROZAC
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = LILLY
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 20MG BASE/5ML
----
Product id = 27061
Application Number = 20974
Date of Application = 9,, Mar, 1999
RX/OTC/DISCN = DISCN
Tradename = PROZAC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LILLY
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27062
Application Number = 20974
Date of Application = 9,, Mar, 1999
RX/OTC/DISCN = DISCN
Tradename = PROZAC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LILLY
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 242
Application Number = 21235
Date of Application = 26,, Feb, 2001
RX/OTC/DISCN = RX
Tradename = PROZAC WEEKLY
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = N
Applicant Name = LILLY
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 90MG BASE
----
Product id = 3363
Application Number = 21520
Date of Application = 9,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = SYMBYAX
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = LILLY
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 25MG BASE;EQ 3MG BASE
----
Product id = 3364
Application Number = 21520
Date of Application = 24,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = SYMBYAX
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = LILLY
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 25MG BASE;EQ 6MG BASE
----
Product id = 3366
Application Number = 21520
Date of Application = 24,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = SYMBYAX
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = LILLY
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = EQ 50MG BASE;EQ 6MG BASE
----
Product id = 3365
Application Number = 21520
Date of Application = 24,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = SYMBYAX
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = LILLY
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 25MG BASE;EQ 12MG BASE
----
Product id = 3367
Application Number = 21520
Date of Application = 24,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = SYMBYAX
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = LILLY
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 50MG BASE;EQ 12MG BASE
----
Product id = 27833
Application Number = 21860
Date of Application = 19,, May, 2006
RX/OTC/DISCN = RX
Tradename = SARAFEM
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WARNER CHILCOTT LLC
ProductNo = 001
Tecode = AB1
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27834
Application Number = 21860
Date of Application = 19,, May, 2006
RX/OTC/DISCN = RX
Tradename = SARAFEM
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WARNER CHILCOTT LLC
ProductNo = 002
Tecode = AB1
Rld = No
Strength = EQ 15MG BASE
----
Product id = 27835
Application Number = 21860
Date of Application = 19,, May, 2006
RX/OTC/DISCN = RX
Tradename = SARAFEM
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WARNER CHILCOTT LLC
ProductNo = 003
Tecode = AB1
Rld = Yes
Strength = EQ 20MG BASE
----
Product id = 1970
Application Number = 74803
Date of Application = 2,, Aug, 2001
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB1
Rld = No
Strength = EQ 20MG BASE
----
Product id = 1969
Application Number = 74803
Date of Application = 30,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = BARR
ProductNo = 002
Tecode = AB1
Rld = No
Strength = EQ 10MG BASE
----
Product id = 2000
Application Number = 75049
Date of Application = 2,, Aug, 2001
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB1
Rld = No
Strength = EQ 10MG BASE
----
Product id = 2003
Application Number = 75049
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB1
Rld = No
Strength = EQ 20MG BASE
----
Product id = 2006
Application Number = 75049
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 1989
Application Number = 75207
Date of Application = 30,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB1
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1991
Application Number = 75207
Date of Application = 30,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB1
Rld = No
Strength = EQ 20MG BASE
----
Product id = 1993
Application Number = 75207
Date of Application = 25,, May, 2007
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 1983
Application Number = 75245
Date of Application = 31,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB1
Rld = No
Strength = EQ 20MG BASE
----
Product id = 1982
Application Number = 75245
Date of Application = 31,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB1
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1984
Application Number = 75245
Date of Application = 28,, Sep, 2004
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 13808
Application Number = 75292
Date of Application = 7,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 20MG BASE/5ML
----
Product id = 2007
Application Number = 75452
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB1
Rld = No
Strength = EQ 10MG BASE
----
Product id = 2008
Application Number = 75452
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB1
Rld = No
Strength = EQ 20MG BASE
----
Product id = 2009
Application Number = 75452
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 1985
Application Number = 75464
Date of Application = 30,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LANDELA PHARM
ProductNo = 001
Tecode = AB1
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1986
Application Number = 75464
Date of Application = 30,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LANDELA PHARM
ProductNo = 002
Tecode = AB1
Rld = No
Strength = EQ 20MG BASE
----
Product id = 1976
Application Number = 75465
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB1
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1977
Application Number = 75465
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB1
Rld = No
Strength = EQ 20MG BASE
----
Product id = 1978
Application Number = 75465
Date of Application = 2,, Aug, 2001
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 13815
Application Number = 75506
Date of Application = 2,, Aug, 2001
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 20MG BASE/5ML
----
Product id = 13816
Application Number = 75514
Date of Application = 29,, Aug, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 20MG BASE/5ML
----
Product id = 13810
Application Number = 75525
Date of Application = 27,, Jun, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = HI TECH PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 20MG BASE/5ML
----
Product id = 1994
Application Number = 75577
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB1
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1995
Application Number = 75577
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB1
Rld = No
Strength = EQ 20MG BASE
----
Product id = 1987
Application Number = 75658
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 001
Tecode = AB1
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1988
Application Number = 75658
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 002
Tecode = AB1
Rld = No
Strength = EQ 20MG BASE
----
Product id = 1959
Application Number = 75662
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1960
Application Number = 75662
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 13807
Application Number = 75690
Date of Application = 31,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 20MG BASE/5ML
----
Product id = 21434
Application Number = 75755
Date of Application = 2,, Aug, 2001
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 21435
Application Number = 75755
Date of Application = 2,, Aug, 2001
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 20MG BASE
----
Product id = 1957
Application Number = 75787
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MUTUAL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1958
Application Number = 75787
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MUTUAL PHARMA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 2001
Application Number = 75807
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 2004
Application Number = 75807
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 21429
Application Number = 75810
Date of Application = 1,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 21432
Application Number = 75865
Date of Application = 28,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 21433
Application Number = 75865
Date of Application = 30,, Aug, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 40MG BASE
----
Product id = 21437
Application Number = 75872
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 13812
Application Number = 75920
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 20MG BASE/5ML
----
Product id = 2010
Application Number = 76001
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB1
Rld = No
Strength = EQ 10MG BASE
----
Product id = 2011
Application Number = 76001
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AB1
Rld = No
Strength = EQ 20MG BASE
----
Product id = 21430
Application Number = 76006
Date of Application = 30,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 13813
Application Number = 76015
Date of Application = 30,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = PHARM ASSOC
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = EQ 20MG BASE/5ML
----
Product id = 1972
Application Number = 76022
Date of Application = 30,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = CARLSBAD
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1973
Application Number = 76022
Date of Application = 30,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = CARLSBAD
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 21436
Application Number = 76024
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1974
Application Number = 76165
Date of Application = 1,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = CR DOUBLE CRANE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1975
Application Number = 76165
Date of Application = 1,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = CR DOUBLE CRANE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 181
Application Number = 76237
Date of Application = 24,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 90MG BASE
----
Product id = 1971
Application Number = 76251
Date of Application = 18,, May, 2005
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 40MG BASE
----
Product id = 1964
Application Number = 76287
Date of Application = 20,, May, 2008
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode = AB2
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1965
Application Number = 76287
Date of Application = 20,, May, 2008
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 002
Tecode = AB2
Rld = No
Strength = EQ 20MG BASE
----
Product id = 13811
Application Number = 76458
Date of Application = 14,, May, 2004
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 20MG BASE/5ML
----
Product id = 1996
Application Number = 76922
Date of Application = 16,, Dec, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1997
Application Number = 76922
Date of Application = 16,, Dec, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 1998
Application Number = 76922
Date of Application = 16,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 1999
Application Number = 76990
Date of Application = 13,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 2002
Application Number = 77469
Date of Application = 17,, Nov, 2008
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB2
Rld = No
Strength = EQ 10MG BASE
----
Product id = 2005
Application Number = 77469
Date of Application = 17,, Nov, 2008
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB2
Rld = No
Strength = EQ 20MG BASE
----
Product id = 2698
Application Number = 77528
Date of Application = 19,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = OLANZAPINE AND FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 25MG BASE;EQ 6MG BASE
----
Product id = 2699
Application Number = 77528
Date of Application = 19,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = OLANZAPINE AND FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 25MG BASE;EQ 12MG BASE
----
Product id = 2700
Application Number = 77528
Date of Application = 19,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = OLANZAPINE AND FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 50MG BASE;EQ 6MG BASE
----
Product id = 2701
Application Number = 77528
Date of Application = 19,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = OLANZAPINE AND FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 50MG BASE;EQ 12MG BASE
----
Product id = 2687
Application Number = 77742
Date of Application = 2,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = OLANZAPINE AND FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 25MG BASE;EQ 3MG BASE
----
Product id = 2688
Application Number = 77742
Date of Application = 2,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = OLANZAPINE AND FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 25MG BASE;EQ 6MG BASE
----
Product id = 2689
Application Number = 77742
Date of Application = 2,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = OLANZAPINE AND FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 25MG BASE;EQ 12MG BASE
----
Product id = 2690
Application Number = 77742
Date of Application = 2,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = OLANZAPINE AND FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 50MG BASE;EQ 6MG BASE
----
Product id = 2691
Application Number = 77742
Date of Application = 2,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = OLANZAPINE AND FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 50MG BASE;EQ 12MG BASE
----
Product id = 13814
Application Number = 77849
Date of Application = 9,, Feb, 2007
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = SILARX
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 20MG BASE/5ML
----
Product id = 1990
Application Number = 78045
Date of Application = 17,, Nov, 2008
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB2
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1992
Application Number = 78045
Date of Application = 17,, Nov, 2008
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB2
Rld = No
Strength = EQ 20MG BASE
----
Product id = 2012
Application Number = 78143
Date of Application = 16,, Jan, 2008
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 2013
Application Number = 78143
Date of Application = 16,, Jan, 2008
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 2014
Application Number = 78143
Date of Application = 16,, Jan, 2008
RX/OTC/DISCN = DISCN
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 40MG BASE
----
Product id = 182
Application Number = 78572
Date of Application = 22,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 90MG BASE
----
Product id = 1966
Application Number = 78619
Date of Application = 31,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB1
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1967
Application Number = 78619
Date of Application = 31,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB1
Rld = No
Strength = EQ 20MG BASE
----
Product id = 1968
Application Number = 78619
Date of Application = 31,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 2693
Application Number = 78901
Date of Application = 16,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = OLANZAPINE AND FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 25MG BASE;EQ 6MG BASE
----
Product id = 2695
Application Number = 78901
Date of Application = 16,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = OLANZAPINE AND FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 50MG BASE;EQ 6MG BASE
----
Product id = 2694
Application Number = 78901
Date of Application = 16,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = OLANZAPINE AND FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 25MG BASE;EQ 12MG BASE
----
Product id = 2696
Application Number = 78901
Date of Application = 16,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = OLANZAPINE AND FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 50MG BASE;EQ 12MG BASE
----
Product id = 2692
Application Number = 78901
Date of Application = 16,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = OLANZAPINE AND FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 25MG BASE;EQ 3MG BASE
----
Product id = 13809
Application Number = 79209
Date of Application = 20,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = AUROBINDO PHARM
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 20MG BASE/5ML
----
Product id = 1961
Application Number = 90223
Date of Application = 19,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 001
Tecode = AB1
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1962
Application Number = 90223
Date of Application = 19,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 002
Tecode = AB1
Rld = No
Strength = EQ 20MG BASE
----
Product id = 1963
Application Number = 90223
Date of Application = 19,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 27860
Application Number = 200151
Date of Application = 3,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = SELFEMRA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB1
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27861
Application Number = 200151
Date of Application = 3,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = SELFEMRA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AB1
Rld = No
Strength = EQ 15MG BASE
----
Product id = 27862
Application Number = 200151
Date of Application = 3,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = SELFEMRA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 003
Tecode = AB1
Rld = No
Strength = EQ 20MG BASE
----
Product id = 1979
Application Number = 201336
Date of Application = 1,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 001
Tecode = AB1
Rld = No
Strength = EQ 10MG BASE
----
Product id = 1980
Application Number = 201336
Date of Application = 1,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 002
Tecode = AB1
Rld = No
Strength = EQ 20MG BASE
----
Product id = 1981
Application Number = 201336
Date of Application = 1,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 2697
Application Number = 202074
Date of Application = 25,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = OLANZAPINE AND FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 25MG BASE;EQ 3MG BASE
----
Product id = 21431
Application Number = 202133
Date of Application = 6,, Oct, 2011
RX/OTC/DISCN = RX
Tradename = FLUOXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = EDGEMONT PHARMS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 60MG BASE
----