fluvoxamine-maleate
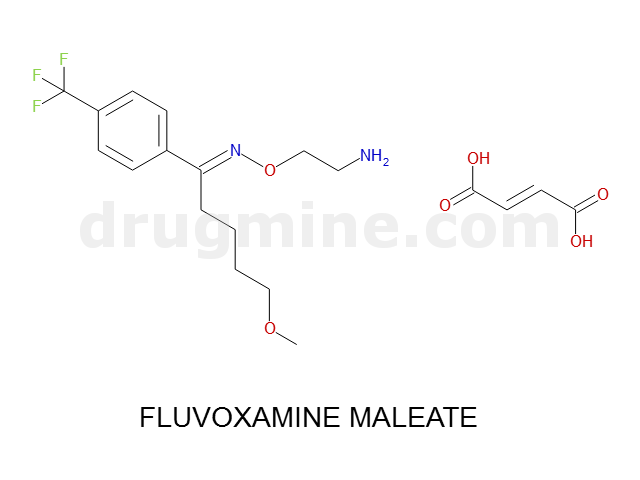
Name: FLUVOXAMINE MALEATE
ID :
MW: 318
Number of atoms: 22
Molecular_Formula: C15H21F3N2O2
Alogp: 2.551
Indication class : Antiobsessional Agent
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
fluvoxamine maleate containing products summary
There are in total 53 different products containing the active ingredient fluvoxamine maleate. From the 53 drug products, 27 have been discontinued.Product id = 24096
Application Number = 20243
Date of Application = 5,, Dec, 1994
RX/OTC/DISCN = DISCN
Tradename = LUVOX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 24097
Application Number = 20243
Date of Application = 5,, Dec, 1994
RX/OTC/DISCN = DISCN
Tradename = LUVOX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SOLVAY
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 24098
Application Number = 20243
Date of Application = 5,, Dec, 1994
RX/OTC/DISCN = DISCN
Tradename = LUVOX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SOLVAY
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 24099
Application Number = 20243
Date of Application = 5,, Dec, 1994
RX/OTC/DISCN = DISCN
Tradename = LUVOX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SOLVAY
ProductNo = 004
Tecode =
Rld = No
Strength = 150MG
----
Product id = 24093
Application Number = 21519
Date of Application = 20,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = LUVOX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ANI PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 24094
Application Number = 21519
Date of Application = 20,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = LUVOX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ANI PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 24095
Application Number = 21519
Date of Application = 20,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = LUVOX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ANI PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 591
Application Number = 22033
Date of Application = 28,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = LUVOX CR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = JAZZ PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 592
Application Number = 22033
Date of Application = 28,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = LUVOX CR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = JAZZ PHARMS
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 150MG
----
Product id = 21493
Application Number = 75887
Date of Application = 5,, Jan, 2001
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 21495
Application Number = 75887
Date of Application = 5,, Jan, 2001
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21497
Application Number = 75887
Date of Application = 5,, Jan, 2001
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21494
Application Number = 75888
Date of Application = 29,, Nov, 2000
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 21496
Application Number = 75888
Date of Application = 29,, Nov, 2000
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21498
Application Number = 75888
Date of Application = 29,, Nov, 2000
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 100MG
----
Product id = 21488
Application Number = 75889
Date of Application = 29,, Nov, 2000
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 21489
Application Number = 75889
Date of Application = 29,, Nov, 2000
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21491
Application Number = 75889
Date of Application = 29,, Nov, 2000
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21505
Application Number = 75893
Date of Application = 10,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 21506
Application Number = 75893
Date of Application = 10,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21507
Application Number = 75893
Date of Application = 10,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21508
Application Number = 75894
Date of Application = 18,, Apr, 2001
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 21509
Application Number = 75894
Date of Application = 18,, Apr, 2001
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21510
Application Number = 75894
Date of Application = 18,, Apr, 2001
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21479
Application Number = 75897
Date of Application = 25,, Jan, 2001
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 21480
Application Number = 75897
Date of Application = 25,, Jan, 2001
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21481
Application Number = 75897
Date of Application = 25,, Jan, 2001
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21482
Application Number = 75898
Date of Application = 12,, Mar, 2001
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 21483
Application Number = 75898
Date of Application = 12,, Mar, 2001
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21484
Application Number = 75898
Date of Application = 12,, Mar, 2001
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21502
Application Number = 75899
Date of Application = 17,, Jan, 2001
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SYNTHON PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 21503
Application Number = 75899
Date of Application = 17,, Jan, 2001
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SYNTHON PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21504
Application Number = 75899
Date of Application = 17,, Jan, 2001
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SYNTHON PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21499
Application Number = 75900
Date of Application = 23,, Feb, 2006
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 21500
Application Number = 75900
Date of Application = 23,, Feb, 2006
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21501
Application Number = 75900
Date of Application = 23,, Feb, 2006
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21473
Application Number = 75901
Date of Application = 28,, Dec, 2000
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 21474
Application Number = 75901
Date of Application = 28,, Dec, 2000
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21475
Application Number = 75901
Date of Application = 28,, Dec, 2000
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21476
Application Number = 75902
Date of Application = 7,, May, 2001
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 21477
Application Number = 75902
Date of Application = 7,, May, 2001
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21478
Application Number = 75902
Date of Application = 7,, May, 2001
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21490
Application Number = 75950
Date of Application = 15,, Oct, 2001
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21492
Application Number = 75950
Date of Application = 15,, Oct, 2001
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21485
Application Number = 76125
Date of Application = 29,, Apr, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 21486
Application Number = 76125
Date of Application = 29,, Apr, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21487
Application Number = 76125
Date of Application = 29,, Apr, 2002
RX/OTC/DISCN = DISCN
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 518
Application Number = 91476
Date of Application = 13,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ANCHEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 519
Application Number = 91476
Date of Application = 13,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ANCHEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 516
Application Number = 91482
Date of Application = 23,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 517
Application Number = 91482
Date of Application = 18,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 520
Application Number = 203240
Date of Application = 31,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 521
Application Number = 203240
Date of Application = 31,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = FLUVOXAMINE MALEATE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 150MG
----