folic-acid
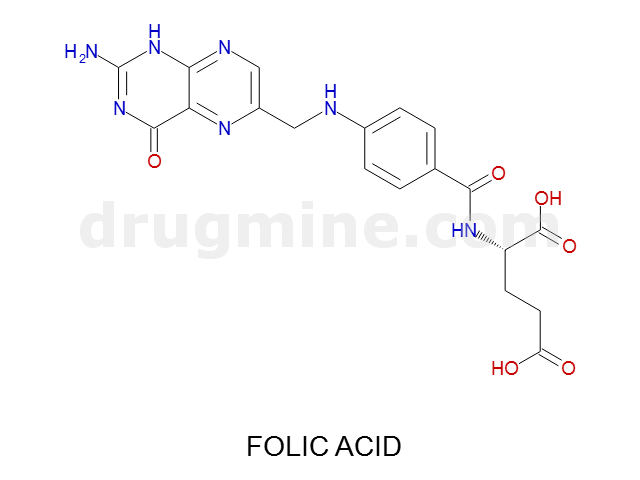
Name: FOLIC ACID
ID :
MW: 441
Number of atoms: 32
Molecular_Formula: C19H19N7O6
Alogp: -0.232
Indication class : Vitamin (hematopoietic)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
folic acid containing products summary
There are in total 54 different products containing the active ingredient folic acid. From the 54 drug products, 36 have been discontinued.Product id = 21548
Application Number = 5897
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLVITE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = HIKMA (MAPLE)
ProductNo = 004
Tecode =
Rld = No
Strength = 1MG
----
Product id = 7745
Application Number = 5897
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLVITE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HIKMA (MAPLE)
ProductNo = 008
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 2048
Application Number = 6012
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLVRON
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = LEDERLE
ProductNo = 003
Tecode =
Rld = No
Strength = 182MG;0.33MG
----
Product id = 6108
Application Number = 6071
Date of Application = 10,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = BEROCCA PN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ROCHE
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG/ML;0.03MG/ML;0.0025MG/ML;7.5MG/ML;100 IU/ML;0.2MG/ML;20MG/ML;2MG/ML;1.8MG/ML;1.5MG/ML;1,650 IU/ML;5 IU/ML
----
Product id = 21528
Application Number = 6135
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LILLY
ProductNo = 003
Tecode =
Rld = No
Strength = 1MG
----
Product id = 8983
Application Number = 8809
Date of Application = 8,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = M.V.I.-12
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 004
Tecode =
Rld = No
Strength = 10MG/ML;0.006MG/ML;0.5MCG/ML;1.5MG/ML;20 IU/ML;0.04MG/ML;4MG/ML;0.4MG/ML;0.36MG/ML;0.3MG/ML;330 UNITS/ML;1 IU/ML
----
Product id = 8984
Application Number = 8809
Date of Application = 22,, Apr, 2004
RX/OTC/DISCN = DISCN
Tradename = M.V.I.-12
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 005
Tecode =
Rld = No
Strength = 20MG/ML;0.006MG/ML;0.5MCG/ML;1.5MG/ML;20 IU/ML;0.6MG/ML;4MG/ML;0.4MG/ML;0.36MG/ML;0.6MG/ML;330 UNITS/ML;1 IU/ML
----
Product id = 8985
Application Number = 8809
Date of Application = 9,, Sep, 2004
RX/OTC/DISCN = RX
Tradename = M.V.I.-12 (WITHOUT VITAMIN K)
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 006
Tecode =
Rld = Yes
Strength = 20MG/ML;0.006MG/ML;0.05MCG/ML;1.5MG/ML;0.0005MG/ML;0.06MG/ML;4MG/ML;0.6MG/ML;0.36MG/ML;0.6MG/ML;0.1MG/ML;1MG/ML
----
Product id = 9479
Application Number = 18439
Date of Application = 8,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = MVC PLUS
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG/ML;0.006MG/ML;0.5MCG/ML;1.5MG/ML;20 IU/ML;0.04MG/ML;4MG/ML;0.4MG/ML;0.36MG/ML;0.3MG/ML;330 UNITS/ML;1 IU/ML
----
Product id = 8982
Application Number = 18440
Date of Application = 8,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = M.V.C. 9+3
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ABRAXIS PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG/ML;0.006MG/ML;0.5MCG/ML;1.5MG/ML;20 IU/ML;0.04MG/ML;4MG/ML;0.4MG/ML;0.36MG/ML;0.3MG/ML;330 UNITS/ML;1 IU/ML
----
Product id = 4818
Application Number = 18920
Date of Application = 21,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = M.V.I. PEDIATRIC
Route/format = IV (INFUSION) / FOR SOLUTION
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 80MG/VIAL;0.02MG/VIAL;0.001MG/VIAL;5MG/VIAL;0.01MG/VIAL;0.14MG/VIAL;17MG/VIAL;0.2MG/VIAL;1MG/VIAL;1.4MG/VIAL;EQ 1.2MG BASE/VIAL;0.7MG/VIAL;7MG/VIAL
----
Product id = 8986
Application Number = 18933
Date of Application = 8,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = M.V.I.-12 LYOPHILIZED
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG/VIAL;0.06MG/VIAL;0.005MG/VIAL;15MG/VIAL;5MCG/VIAL;0.4MG/VIAL;40MG/VIAL;4MG/VIAL;3.6MG/VIAL;3MG/VIAL;1MG/VIAL;10MG/VIAL
----
Product id = 11417
Application Number = 20176
Date of Application = 29,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = VITAPED
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = N/A,80MG/VIAL;N/A,0.02MG/VIAL;N/A,0.001MG/VIAL;400 IU/10ML,N/A;N/A,0.14MG/VIAL;N/A,17MG/VIAL;N/A,5MG/VIAL;0.2MG/10ML,N/A;N/A,1MG/VIAL;N/A,1.4MG/VIAL;N/A,1.2MG/VIAL;EQ 2,300 UNITS BASE/10ML,N/A;7 IU/10ML,N/A
----
Product id = 8408
Application Number = 21163
Date of Application = 18,, May, 2000
RX/OTC/DISCN = RX
Tradename = INFUVITE ADULT
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2 IU/ML;40MG/ML;12MCG/ML;40 IU/ML;1MCG/ML;3MG/ML;120MCG/ML;8MG/ML;1.2MG/ML;0.72MG/ML;1.2MG/ML;660 IU/ML;0.03MG/ML
----
Product id = 11831
Application Number = 21265
Date of Application = 21,, Feb, 2001
RX/OTC/DISCN = RX
Tradename = INFUVITE PEDIATRIC
Route/format = IV (INFUSION) / INJECTABLE
Application Type = N
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = Yes
Strength = 80MG/VIAL;0.02MG/VIAL;400 IU/VIAL;0.001MG/VIAL;5MG/VIAL;0.14MG/VIAL;17MG/VIAL;1MG/VIAL;1.4MG/VIAL;1.2MG/VIAL;7 IU/VIAL;2,300 IU/VIAL;0.2MG/VIAL
----
Product id = 11830
Application Number = 21559
Date of Application = 16,, Jun, 2003
RX/OTC/DISCN = RX
Tradename = INFUVITE ADULT
Route/format = IV (INFUSION) / INJECTABLE
Application Type = N
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2 IU/ML;40MG/ML;12MCG/ML;40 IU/ML;1MCG/ML;3MG/ML;120MCG/ML;8MG/ML;1.2MG/ML;0.72MG/ML;1.2MG/ML;660 IU/ML;30MCG/ML
----
Product id = 11846
Application Number = 21625
Date of Application = 30,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = M.V.I. ADULT
Route/format = IV (INFUSION) / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 200MG/VIAL;0.06MG/VIAL;0.005MG/VIAL;15MG/VIAL;0.005MG/VIAL;0.6MG/VIAL;40MG/VIAL;6MG/VIAL;3.6MG/VIAL;6MG/VIAL;1MG/VIAL;10MG/VIAL;0.15MG/VIAL
----
Product id = 11847
Application Number = 21643
Date of Application = 18,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = M.V.I. ADULT (PHARMACY BULK PACKAGE)
Route/format = IV (INFUSION) / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 200MG/5ML;0.06MG/5ML;0.005MG/5ML;15MG/5ML;0.005MG/5ML;0.6MG/5ML;40MG/5ML;6MG/5ML;3.6MG/5ML;6MG/5ML;1MG/5ML;10MG/5ML;0.15MG/5ML
----
Product id = 11832
Application Number = 21646
Date of Application = 29,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = INFUVITE PEDIATRIC (PHARMACY BULK PACKAGE)
Route/format = IV (INFUSION) / INJECTABLE
Application Type = N
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = Yes
Strength = 80MG/VIAL;0.02MG/VIAL;400 IU/VIAL;0.001MG/VIAL;5MG/VIAL;0.14MG/VIAL;17MG/VIAL;1MG/VIAL;1.4MG/VIAL;1.2MG/VIAL;7 IU/VIAL;2,300 IU/VIAL;0.2MG/VIAL
----
Product id = 21526
Application Number = 40514
Date of Application = 14,, Jun, 2005
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = JUBILANT CADISTA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21530
Application Number = 40582
Date of Application = 18,, Jul, 2005
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21514
Application Number = 40625
Date of Application = 21,, Jul, 2005
RX/OTC/DISCN = RX
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARM
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 1MG
----
Product id = 21541
Application Number = 40756
Date of Application = 4,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG
----
Product id = 21520
Application Number = 40796
Date of Application = 12,, Jan, 2009
RX/OTC/DISCN = RX
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EXCELLIUM
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG
----
Product id = 21522
Application Number = 80600
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG
----
Product id = 21543
Application Number = 80680
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 1MG
----
Product id = 21523
Application Number = 80686
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21546
Application Number = 80691
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WHITEWORTH TOWN PLSN
ProductNo = 002
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21519
Application Number = 80755
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EVERYLIFE
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21534
Application Number = 80784
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21527
Application Number = 80816
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21539
Application Number = 80903
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VALEANT PHARM INTL
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 7742
Application Number = 81066
Date of Application = 29,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEN VENUE
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 21525
Application Number = 83000
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21536
Application Number = 83133
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TABLICAPS
ProductNo = 002
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21544
Application Number = 83141
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21529
Application Number = 83526
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MK LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21521
Application Number = 83598
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21532
Application Number = 84158
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PHARMERAL
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21535
Application Number = 84472
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21531
Application Number = 84915
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NEXGEN PHARMA INC
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21518
Application Number = 85061
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CONTRACT PHARMACAL
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG
----
Product id = 21545
Application Number = 85141
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21542
Application Number = 86296
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21547
Application Number = 87438
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FOLICET
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MISSION PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21538
Application Number = 87828
Date of Application = 13,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21537
Application Number = 88199
Date of Application = 29,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UDL
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21540
Application Number = 88730
Date of Application = 23,, Mar, 1984
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VANGARD
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21533
Application Number = 88949
Date of Application = 13,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PIONEER PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21515
Application Number = 89177
Date of Application = 8,, Jan, 1986
RX/OTC/DISCN = DISCN
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 7743
Application Number = 89202
Date of Application = 18,, Feb, 1986
RX/OTC/DISCN = RX
Tradename = FOLIC ACID
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 5MG/ML
----
Product id = 21524
Application Number = 90035
Date of Application = 9,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG
----
Product id = 21516
Application Number = 91145
Date of Application = 12,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BICON PHARM
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG
----
Product id = 21517
Application Number = 202437
Date of Application = 27,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = FOLIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CADILA PHARMS LTD
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1 MG
----