fosphenytoin-sodium
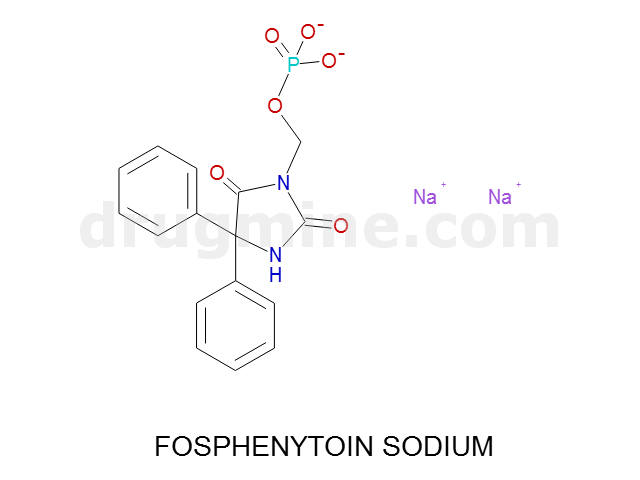

Name: FOSPHENYTOIN SODIUM
ID :
MW: 360
Number of atoms: 25
Molecular_Formula: C16H13N2O6P
Alogp: -0.495
Indication class : Anticonvulsant
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
fosphenytoin sodium containing products summary
There are in total 14 different products containing the active ingredient fosphenytoin sodium. From the 14 drug products, 2 have been discontinued.Product id = 6589
Application Number = 20450
Date of Application = 5,, Aug, 1996
RX/OTC/DISCN = RX
Tradename = CEREBYX
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 50MG PHENYTOIN NA/ML
----
Product id = 7770
Application Number = 76886
Date of Application = 6,, Aug, 2007
RX/OTC/DISCN = DISCN
Tradename = FOSPHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 50MG PHENYTOIN NA/ML
----
Product id = 7762
Application Number = 77481
Date of Application = 6,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = FOSPHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 50MG PHENYTOIN NA/ML
----
Product id = 7765
Application Number = 77989
Date of Application = 6,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = FOSPHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 50MG PHENYTOIN NA/ML
----
Product id = 7763
Application Number = 78052
Date of Application = 6,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = FOSPHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 50MG PHENYTOIN NA/ML
----
Product id = 7761
Application Number = 78126
Date of Application = 6,, Aug, 2007
RX/OTC/DISCN = DISCN
Tradename = FOSPHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 50MG PHENYTOIN NA/ML
----
Product id = 7771
Application Number = 78137
Date of Application = 6,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = FOSPHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 50MG PHENYTOIN NA/ML
----
Product id = 7766
Application Number = 78158
Date of Application = 6,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = FOSPHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 50MG PHENYTOIN NA/ML
----
Product id = 7767
Application Number = 78277
Date of Application = 6,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = FOSPHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 50MG PHENYTOIN NA/ML
----
Product id = 7769
Application Number = 78417
Date of Application = 18,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = FOSPHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 50MG PHENYTOIN NA/ML
----
Product id = 7760
Application Number = 78476
Date of Application = 18,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = FOSPHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AMNEAL PHARMS CO
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 50MG PHENYTOIN NA/ML
----
Product id = 7759
Application Number = 78736
Date of Application = 8,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = FOSPHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 50MG PHENYTOIN NA/ML
----
Product id = 7764
Application Number = 78765
Date of Application = 2,, Dec, 2009
RX/OTC/DISCN = RX
Tradename = FOSPHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 50MG PHENYTOIN NA/ML
----
Product id = 7768
Application Number = 90099
Date of Application = 13,, May, 2010
RX/OTC/DISCN = RX
Tradename = FOSPHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 50MG PHENYTOIN NA/ML
----