furosemide
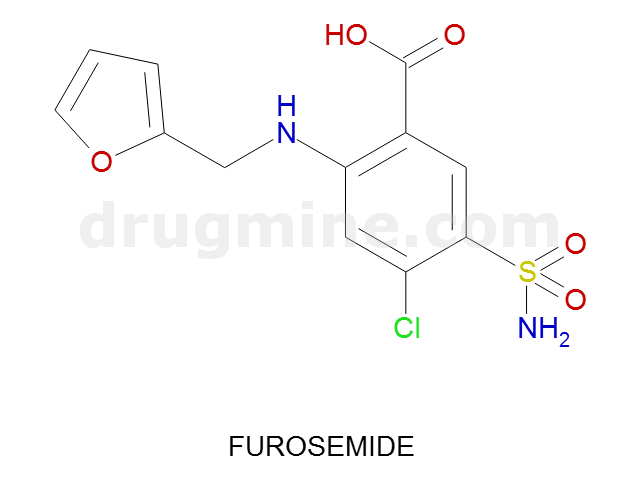

Name: FUROSEMIDE
ID :
MW: 331
Number of atoms: 21
Molecular_Formula: C12H11ClN2O5S
Alogp: 1.403
Indication class : Diuretic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
furosemide containing products summary
There are in total 82 different products containing the active ingredient furosemide. From the 82 drug products, 46 have been discontinued.Product id = 23347
Application Number = 16273
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = LASIX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 23346
Application Number = 16273
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = LASIX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 23348
Application Number = 16273
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = LASIX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 80MG
----
Product id = 8694
Application Number = 16363
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LASIX
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 13876
Application Number = 17688
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LASIX
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 7798
Application Number = 18025
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = INTL MEDICATION
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 7792
Application Number = 18267
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 21660
Application Number = 18369
Date of Application = 14,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 21663
Application Number = 18369
Date of Application = 14,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 40MG
----
Product id = 21650
Application Number = 18370
Date of Application = 10,, Feb, 1983
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 21649
Application Number = 18370
Date of Application = 26,, Jun, 1984
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SUPERPHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG
----
Product id = 21629
Application Number = 18413
Date of Application = 30,, Nov, 1983
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 21630
Application Number = 18413
Date of Application = 30,, Nov, 1983
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 21618
Application Number = 18415
Date of Application = 27,, Jul, 1982
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 21619
Application Number = 18415
Date of Application = 27,, Jul, 1982
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = DAVA PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 40MG
----
Product id = 21620
Application Number = 18415
Date of Application = 26,, Nov, 1984
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = DAVA PHARMS INC
ProductNo = 003
Tecode =
Rld = No
Strength = 80MG
----
Product id = 21654
Application Number = 18419
Date of Application = 31,, Jan, 1983
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 21655
Application Number = 18419
Date of Application = 31,, Jan, 1983
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WARNER CHILCOTT
ProductNo = 002
Tecode =
Rld = No
Strength = 40MG
----
Product id = 21656
Application Number = 18419
Date of Application = 13,, Nov, 1984
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WARNER CHILCOTT
ProductNo = 003
Tecode =
Rld = No
Strength = 80MG
----
Product id = 7804
Application Number = 18420
Date of Application = 26,, Feb, 1982
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 21636
Application Number = 18487
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 21637
Application Number = 18487
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 7783
Application Number = 18507
Date of Application = 30,, Jul, 1982
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 21643
Application Number = 18569
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 21642
Application Number = 18569
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 21645
Application Number = 18569
Date of Application = 14,, Aug, 1984
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANDOZ
ProductNo = 005
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 7799
Application Number = 18579
Date of Application = 30,, Nov, 1983
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = LUITPOLD
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 10MG/ML
----
Product id = 7797
Application Number = 18667
Date of Application = 28,, May, 1982
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 10MG/ML
----
Product id = 7808
Application Number = 18670
Date of Application = 20,, Jul, 1982
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 21644
Application Number = 18750
Date of Application = 30,, Jul, 1984
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 40MG
----
Product id = 21624
Application Number = 18753
Date of Application = 28,, Feb, 1984
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = INTL MEDICATION
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 21625
Application Number = 18753
Date of Application = 28,, Feb, 1984
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = INTL MEDICATION
ProductNo = 002
Tecode =
Rld = No
Strength = 40MG
----
Product id = 21634
Application Number = 18790
Date of Application = 29,, Nov, 1983
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 21639
Application Number = 18823
Date of Application = 10,, Nov, 1983
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 21640
Application Number = 18823
Date of Application = 10,, Nov, 1983
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROXANE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 21631
Application Number = 18868
Date of Application = 28,, Jun, 1983
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = KALAPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 21632
Application Number = 18868
Date of Application = 28,, Jun, 1983
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = KALAPHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 40MG
----
Product id = 7790
Application Number = 18902
Date of Application = 22,, May, 1984
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 10MG/ML
----
Product id = 7784
Application Number = 19036
Date of Application = 13,, Aug, 1984
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 7785
Application Number = 70014
Date of Application = 9,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 7801
Application Number = 70017
Date of Application = 15,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ORGANON USA INC
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 7805
Application Number = 70019
Date of Application = 22,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 7802
Application Number = 70023
Date of Application = 5,, Feb, 1986
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SMITH AND NEPHEW
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 21633
Application Number = 70043
Date of Application = 26,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 7803
Application Number = 70078
Date of Application = 5,, Feb, 1986
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SMITH AND NEPHEW
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 21638
Application Number = 70082
Date of Application = 29,, Oct, 1986
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 21641
Application Number = 70086
Date of Application = 24,, Jan, 1986
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 7786
Application Number = 70095
Date of Application = 9,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 7787
Application Number = 70096
Date of Application = 9,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 21635
Application Number = 70100
Date of Application = 26,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 21657
Application Number = 70412
Date of Application = 26,, Feb, 1986
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 21661
Application Number = 70413
Date of Application = 26,, Feb, 1986
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 13819
Application Number = 70433
Date of Application = 22,, Apr, 1987
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG/5ML
----
Product id = 13818
Application Number = 70434
Date of Application = 22,, Apr, 1987
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 10MG/ML
----
Product id = 21658
Application Number = 70449
Date of Application = 22,, Nov, 1985
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 21662
Application Number = 70450
Date of Application = 22,, Nov, 1985
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 21664
Application Number = 70528
Date of Application = 7,, Jan, 1986
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 7793
Application Number = 70578
Date of Application = 8,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 7806
Application Number = 70604
Date of Application = 2,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 13820
Application Number = 70655
Date of Application = 2,, Oct, 1987
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AA
Rld = No
Strength = 10MG/ML
----
Product id = 21659
Application Number = 71379
Date of Application = 2,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 7791
Application Number = 71439
Date of Application = 14,, Sep, 1990
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 21665
Application Number = 71594
Date of Application = 9,, Feb, 1988
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 7794
Application Number = 72080
Date of Application = 13,, Aug, 1991
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 7800
Application Number = 74017
Date of Application = 30,, Jun, 1994
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MARSAM PHARMS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 7795
Application Number = 74337
Date of Application = 31,, Oct, 1994
RX/OTC/DISCN = DISCN
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 7796
Application Number = 75241
Date of Application = 28,, May, 1999
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 10MG/ML
----
Product id = 21651
Application Number = 76796
Date of Application = 26,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 21652
Application Number = 76796
Date of Application = 26,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 21653
Application Number = 76796
Date of Application = 26,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 21621
Application Number = 77293
Date of Application = 9,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EXCELLIUM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 21622
Application Number = 77293
Date of Application = 9,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EXCELLIUM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 21623
Application Number = 77293
Date of Application = 9,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EXCELLIUM
ProductNo = 003
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 7807
Application Number = 77941
Date of Application = 22,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AP
Rld = No
Strength = 10MG/ML
----
Product id = 21626
Application Number = 78010
Date of Application = 18,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 21627
Application Number = 78010
Date of Application = 18,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 21628
Application Number = 78010
Date of Application = 18,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 21646
Application Number = 91258
Date of Application = 1,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 21647
Application Number = 91258
Date of Application = 1,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 21648
Application Number = 91258
Date of Application = 1,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 7788
Application Number = 202747
Date of Application = 27,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CLARIS LIFESCIENCES
ProductNo = 001
Tecode = AP
Rld = No
Strength = 10MG/ML
----
Product id = 7789
Application Number = 203428
Date of Application = 26,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = FUROSEMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EMCURE PHARMS LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 10MG/ML
----