galantamine-hydrobromide
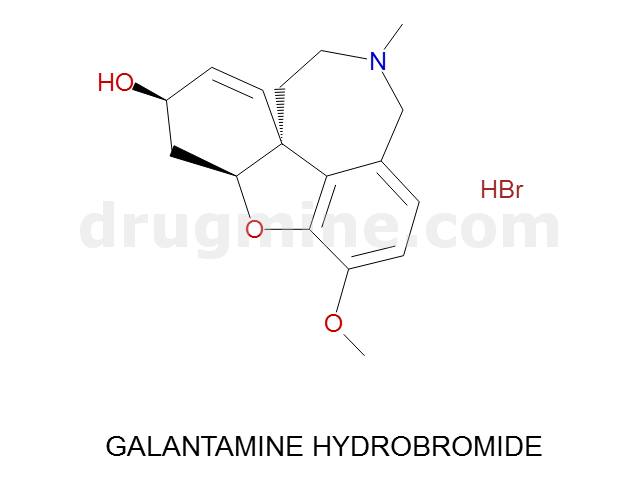

Name: GALANTAMINE HYDROBROMIDE
ID :
MW: 287
Number of atoms: 21
Molecular_Formula: C17H21NO3
Alogp: 1.442
Indication class : .
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
galantamine hydrobromide containing products summary
There are in total 59 different products containing the active ingredient galantamine hydrobromide. From the 59 drug products, 6 have been discontinued.Product id = 27392
Application Number = 21169
Date of Application = 28,, Feb, 2001
RX/OTC/DISCN = RX
Tradename = RAZADYNE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 4MG BASE
----
Product id = 27393
Application Number = 21169
Date of Application = 28,, Feb, 2001
RX/OTC/DISCN = RX
Tradename = RAZADYNE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 27394
Application Number = 21169
Date of Application = 28,, Feb, 2001
RX/OTC/DISCN = RX
Tradename = RAZADYNE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 14016
Application Number = 21224
Date of Application = 22,, Jun, 2001
RX/OTC/DISCN = RX
Tradename = RAZADYNE
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 4MG/ML
----
Product id = 727
Application Number = 21615
Date of Application = 1,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = RAZADYNE ER
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 8MG BASE
----
Product id = 728
Application Number = 21615
Date of Application = 1,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = RAZADYNE ER
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 16MG BASE
----
Product id = 729
Application Number = 21615
Date of Application = 1,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = RAZADYNE ER
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 24MG BASE
----
Product id = 21717
Application Number = 77585
Date of Application = 15,, Sep, 2009
RX/OTC/DISCN = DISCN
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 21718
Application Number = 77585
Date of Application = 15,, Sep, 2009
RX/OTC/DISCN = DISCN
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 8MG BASE
----
Product id = 21719
Application Number = 77585
Date of Application = 15,, Sep, 2009
RX/OTC/DISCN = DISCN
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 12MG BASE
----
Product id = 21744
Application Number = 77587
Date of Application = 9,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 21745
Application Number = 77587
Date of Application = 9,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 21746
Application Number = 77587
Date of Application = 9,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 21741
Application Number = 77589
Date of Application = 22,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 21742
Application Number = 77589
Date of Application = 22,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 21743
Application Number = 77589
Date of Application = 22,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 21732
Application Number = 77590
Date of Application = 29,, May, 2009
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 21734
Application Number = 77590
Date of Application = 29,, May, 2009
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 21736
Application Number = 77590
Date of Application = 29,, May, 2009
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 21729
Application Number = 77593
Date of Application = 11,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 21730
Application Number = 77593
Date of Application = 11,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 21731
Application Number = 77593
Date of Application = 11,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 21733
Application Number = 77603
Date of Application = 28,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 21735
Application Number = 77603
Date of Application = 28,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 21737
Application Number = 77603
Date of Application = 28,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 21747
Application Number = 77604
Date of Application = 6,, Feb, 2009
RX/OTC/DISCN = DISCN
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = YABAO PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 21748
Application Number = 77604
Date of Application = 6,, Feb, 2009
RX/OTC/DISCN = DISCN
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = YABAO PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 8MG BASE
----
Product id = 21749
Application Number = 77604
Date of Application = 6,, Feb, 2009
RX/OTC/DISCN = DISCN
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = YABAO PHARM
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 12MG BASE
----
Product id = 21726
Application Number = 77605
Date of Application = 28,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 21727
Application Number = 77605
Date of Application = 28,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 21728
Application Number = 77605
Date of Application = 28,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 21738
Application Number = 77608
Date of Application = 11,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 21739
Application Number = 77608
Date of Application = 11,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 21740
Application Number = 77608
Date of Application = 11,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 21720
Application Number = 77781
Date of Application = 27,, Sep, 2011
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 21721
Application Number = 77781
Date of Application = 27,, Sep, 2011
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 21722
Application Number = 77781
Date of Application = 27,, Sep, 2011
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 13825
Application Number = 78185
Date of Application = 30,, Jan, 2009
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AA
Rld = No
Strength = 4MG/ML
----
Product id = 530
Application Number = 78189
Date of Application = 15,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 531
Application Number = 78189
Date of Application = 15,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = BARR
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 16MG BASE
----
Product id = 532
Application Number = 78189
Date of Application = 15,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = BARR
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 24MG BASE
----
Product id = 533
Application Number = 78484
Date of Application = 27,, May, 2009
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 534
Application Number = 78484
Date of Application = 27,, May, 2009
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 16MG BASE
----
Product id = 535
Application Number = 78484
Date of Application = 27,, May, 2009
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 24MG BASE
----
Product id = 21750
Application Number = 78898
Date of Application = 17,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 21751
Application Number = 78898
Date of Application = 17,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 21752
Application Number = 78898
Date of Application = 17,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 542
Application Number = 79028
Date of Application = 15,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 543
Application Number = 79028
Date of Application = 15,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 16MG BASE
----
Product id = 544
Application Number = 79028
Date of Application = 15,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 24MG BASE
----
Product id = 539
Application Number = 90178
Date of Application = 2,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 540
Application Number = 90178
Date of Application = 2,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 16MG BASE
----
Product id = 541
Application Number = 90178
Date of Application = 2,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 24MG BASE
----
Product id = 536
Application Number = 90900
Date of Application = 24,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 537
Application Number = 90900
Date of Application = 24,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 16MG BASE
----
Product id = 538
Application Number = 90900
Date of Application = 24,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 24MG BASE
----
Product id = 21723
Application Number = 90957
Date of Application = 29,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 21724
Application Number = 90957
Date of Application = 29,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 21725
Application Number = 90957
Date of Application = 29,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = GALANTAMINE HYDROBROMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----