glimepiride
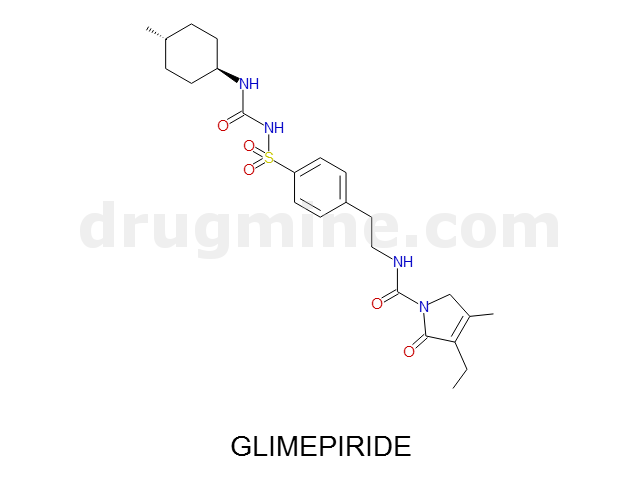


Name: GLIMEPIRIDE
ID :
MW: 491
Number of atoms: 34
Molecular_Formula: C24H34N4O5S
Alogp: 3.781
Indication class : Hypoglycemic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
glimepiride containing products summary
There are in total 67 different products containing the active ingredient glimepiride. From the 67 drug products, 15 have been discontinued.Product id = 17592
Application Number = 20496
Date of Application = 30,, Nov, 1995
RX/OTC/DISCN = RX
Tradename = AMARYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 1MG
----
Product id = 17593
Application Number = 20496
Date of Application = 30,, Nov, 1995
RX/OTC/DISCN = RX
Tradename = AMARYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 17594
Application Number = 20496
Date of Application = 30,, Nov, 1995
RX/OTC/DISCN = RX
Tradename = AMARYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 18227
Application Number = 21700
Date of Application = 23,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = AVANDARYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 001
Tecode =
Rld = Yes
Strength = 1MG;4MG
----
Product id = 18228
Application Number = 21700
Date of Application = 23,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = AVANDARYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 002
Tecode =
Rld = No
Strength = 2MG;4MG
----
Product id = 18230
Application Number = 21700
Date of Application = 23,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = AVANDARYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 003
Tecode =
Rld = No
Strength = 4MG;4MG
----
Product id = 18229
Application Number = 21700
Date of Application = 30,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = AVANDARYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 004
Tecode =
Rld = No
Strength = 2MG;8MG
----
Product id = 18231
Application Number = 21700
Date of Application = 30,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = AVANDARYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 005
Tecode =
Rld = No
Strength = 4MG;8MG
----
Product id = 20760
Application Number = 21925
Date of Application = 28,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = DUETACT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 2MG;30MG
----
Product id = 20761
Application Number = 21925
Date of Application = 28,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = DUETACT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 4MG;30MG
----
Product id = 21828
Application Number = 76802
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 21829
Application Number = 76802
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 21830
Application Number = 76802
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 21824
Application Number = 76875
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = DISCN
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21825
Application Number = 76875
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = DISCN
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 002
Tecode =
Rld = No
Strength = 2MG
----
Product id = 21826
Application Number = 76875
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = DISCN
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 003
Tecode =
Rld = No
Strength = 4MG
----
Product id = 21827
Application Number = 76875
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = DISCN
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 004
Tecode =
Rld = No
Strength = 8MG
----
Product id = 21786
Application Number = 76995
Date of Application = 27,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 21787
Application Number = 76995
Date of Application = 27,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 21788
Application Number = 76995
Date of Application = 27,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 21798
Application Number = 77091
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 21799
Application Number = 77091
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 21800
Application Number = 77091
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 21795
Application Number = 77274
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = DISCN
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21796
Application Number = 77274
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = DISCN
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 002
Tecode =
Rld = No
Strength = 2MG
----
Product id = 21797
Application Number = 77274
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = DISCN
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 003
Tecode =
Rld = No
Strength = 4MG
----
Product id = 21835
Application Number = 77280
Date of Application = 3,, Feb, 2006
RX/OTC/DISCN = DISCN
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21836
Application Number = 77280
Date of Application = 3,, Feb, 2006
RX/OTC/DISCN = DISCN
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 2MG
----
Product id = 21837
Application Number = 77280
Date of Application = 3,, Feb, 2006
RX/OTC/DISCN = DISCN
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 4MG
----
Product id = 21807
Application Number = 77295
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 21808
Application Number = 77295
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 21809
Application Number = 77295
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 21822
Application Number = 77366
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = DISCN
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode =
Rld = No
Strength = 3MG
----
Product id = 21823
Application Number = 77366
Date of Application = 6,, Oct, 2005
RX/OTC/DISCN = DISCN
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode =
Rld = No
Strength = 6MG
----
Product id = 21831
Application Number = 77370
Date of Application = 23,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 21832
Application Number = 77370
Date of Application = 23,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 21833
Application Number = 77370
Date of Application = 23,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 21834
Application Number = 77370
Date of Application = 23,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 004
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 21816
Application Number = 77486
Date of Application = 10,, Feb, 2006
RX/OTC/DISCN = DISCN
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 21818
Application Number = 77486
Date of Application = 10,, Feb, 2006
RX/OTC/DISCN = DISCN
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode =
Rld = No
Strength = 2MG
----
Product id = 21820
Application Number = 77486
Date of Application = 10,, Feb, 2006
RX/OTC/DISCN = DISCN
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode =
Rld = No
Strength = 4MG
----
Product id = 21817
Application Number = 77624
Date of Application = 28,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 21819
Application Number = 77624
Date of Application = 28,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 21821
Application Number = 77624
Date of Application = 28,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 21792
Application Number = 77911
Date of Application = 22,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CARLSBAD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 21793
Application Number = 77911
Date of Application = 22,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CARLSBAD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 21794
Application Number = 77911
Date of Application = 22,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CARLSBAD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 21783
Application Number = 78181
Date of Application = 23,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 21784
Application Number = 78181
Date of Application = 23,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 21785
Application Number = 78181
Date of Application = 23,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 21801
Application Number = 78952
Date of Application = 1,, Aug, 2013
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 21802
Application Number = 78952
Date of Application = 1,, Aug, 2013
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 21803
Application Number = 78952
Date of Application = 1,, Aug, 2013
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 21810
Application Number = 91220
Date of Application = 29,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MICRO LABS LTD INDIA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 21811
Application Number = 91220
Date of Application = 29,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MICRO LABS LTD INDIA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 21812
Application Number = 91220
Date of Application = 29,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MICRO LABS LTD INDIA
ProductNo = 003
Tecode =
Rld = No
Strength = 3MG
----
Product id = 21813
Application Number = 91220
Date of Application = 29,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MICRO LABS LTD INDIA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 21814
Application Number = 91220
Date of Application = 29,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MICRO LABS LTD INDIA
ProductNo = 005
Tecode =
Rld = No
Strength = 6MG
----
Product id = 21815
Application Number = 91220
Date of Application = 29,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MICRO LABS LTD INDIA
ProductNo = 006
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 26296
Application Number = 201049
Date of Application = 4,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG;30MG
----
Product id = 26297
Application Number = 201049
Date of Application = 4,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 4MG;30MG
----
Product id = 21804
Application Number = 202112
Date of Application = 17,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INDOCO REMEDIES
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 21805
Application Number = 202112
Date of Application = 17,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INDOCO REMEDIES
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 21806
Application Number = 202112
Date of Application = 17,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INDOCO REMEDIES
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 21789
Application Number = 202759
Date of Application = 29,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 21790
Application Number = 202759
Date of Application = 29,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 21791
Application Number = 202759
Date of Application = 29,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----