glipizide
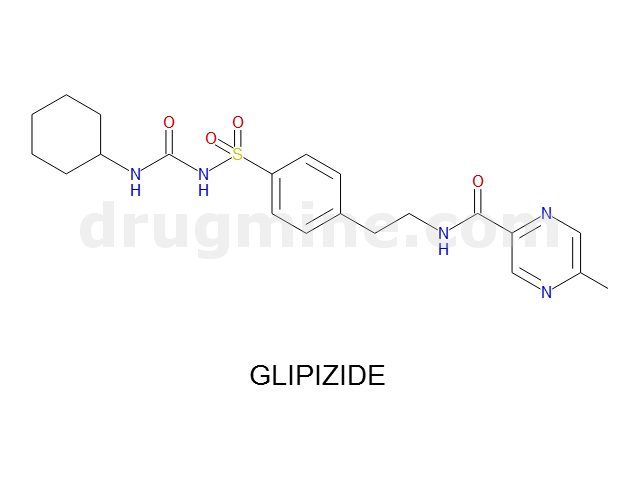

Name: GLIPIZIDE
ID :
MW: 446
Number of atoms: 31
Molecular_Formula: C21H27N5O4S
Alogp: 1.902
Indication class : Antidiabetic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
glipizide containing products summary
There are in total 58 different products containing the active ingredient glipizide. From the 58 drug products, 14 have been discontinued.Product id = 21889
Application Number = 17783
Date of Application = 8,, May, 1984
RX/OTC/DISCN = RX
Tradename = GLUCOTROL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 21890
Application Number = 17783
Date of Application = 8,, May, 1984
RX/OTC/DISCN = RX
Tradename = GLUCOTROL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 10MG
----
Product id = 21888
Application Number = 17783
Date of Application = 11,, May, 1993
RX/OTC/DISCN = DISCN
Tradename = GLUCOTROL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 003
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 15974
Application Number = 20329
Date of Application = 26,, Apr, 1994
RX/OTC/DISCN = RX
Tradename = GLUCOTROL XL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 15975
Application Number = 20329
Date of Application = 26,, Apr, 1994
RX/OTC/DISCN = RX
Tradename = GLUCOTROL XL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 10MG
----
Product id = 15973
Application Number = 20329
Date of Application = 10,, Aug, 1999
RX/OTC/DISCN = RX
Tradename = GLUCOTROL XL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = PFIZER
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 24373
Application Number = 21460
Date of Application = 21,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = METAGLIP
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG;250MG
----
Product id = 24374
Application Number = 21460
Date of Application = 21,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = METAGLIP
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 002
Tecode =
Rld = No
Strength = 2.5MG;500MG
----
Product id = 24375
Application Number = 21460
Date of Application = 21,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = METAGLIP
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 003
Tecode =
Rld = No
Strength = 5MG;500MG
----
Product id = 21860
Application Number = 74223
Date of Application = 27,, Feb, 1995
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 21862
Application Number = 74223
Date of Application = 27,, Feb, 1995
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 21846
Application Number = 74226
Date of Application = 10,, May, 1994
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 21848
Application Number = 74226
Date of Application = 10,, May, 1994
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 21850
Application Number = 74305
Date of Application = 7,, Apr, 1995
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 21852
Application Number = 74305
Date of Application = 7,, Apr, 1995
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 21861
Application Number = 74370
Date of Application = 22,, Nov, 1994
RX/OTC/DISCN = DISCN
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 21863
Application Number = 74370
Date of Application = 22,, Nov, 1994
RX/OTC/DISCN = DISCN
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 21858
Application Number = 74378
Date of Application = 28,, Nov, 1994
RX/OTC/DISCN = DISCN
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 21859
Application Number = 74378
Date of Application = 28,, Nov, 1994
RX/OTC/DISCN = DISCN
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 21856
Application Number = 74387
Date of Application = 4,, Mar, 1996
RX/OTC/DISCN = DISCN
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 21857
Application Number = 74387
Date of Application = 4,, Mar, 1996
RX/OTC/DISCN = DISCN
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 21847
Application Number = 74438
Date of Application = 20,, Jun, 1995
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 21849
Application Number = 74438
Date of Application = 20,, Jun, 1995
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 21844
Application Number = 74497
Date of Application = 31,, Aug, 1995
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 21845
Application Number = 74497
Date of Application = 31,, Aug, 1995
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 21851
Application Number = 74542
Date of Application = 20,, Jun, 1995
RX/OTC/DISCN = DISCN
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 21853
Application Number = 74542
Date of Application = 20,, Jun, 1995
RX/OTC/DISCN = DISCN
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 21838
Application Number = 74550
Date of Application = 11,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 21839
Application Number = 74550
Date of Application = 11,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 21842
Application Number = 74619
Date of Application = 4,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 21843
Application Number = 74619
Date of Application = 4,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR LABS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 21840
Application Number = 75795
Date of Application = 13,, Jun, 2001
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 21841
Application Number = 75795
Date of Application = 13,, Jun, 2001
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 15967
Application Number = 76159
Date of Application = 20,, Sep, 2013
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 15966
Application Number = 76159
Date of Application = 20,, Sep, 2013
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 15969
Application Number = 76467
Date of Application = 8,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 15970
Application Number = 76467
Date of Application = 7,, Nov, 2003
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 15968
Application Number = 76467
Date of Application = 27,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 21876
Application Number = 77270
Date of Application = 28,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;250MG
----
Product id = 21877
Application Number = 77270
Date of Application = 28,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21878
Application Number = 77270
Date of Application = 28,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 5MG;500MG
----
Product id = 21864
Application Number = 77507
Date of Application = 27,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;250MG
----
Product id = 21865
Application Number = 77507
Date of Application = 27,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21866
Application Number = 77507
Date of Application = 27,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;500MG
----
Product id = 21873
Application Number = 77620
Date of Application = 11,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;250MG
----
Product id = 21874
Application Number = 77620
Date of Application = 11,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21875
Application Number = 77620
Date of Application = 11,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;500MG
----
Product id = 21854
Application Number = 77820
Date of Application = 11,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 21855
Application Number = 77820
Date of Application = 11,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 21870
Application Number = 78083
Date of Application = 12,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;250MG
----
Product id = 21871
Application Number = 78083
Date of Application = 12,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21872
Application Number = 78083
Date of Application = 12,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;500MG
----
Product id = 21867
Application Number = 78728
Date of Application = 23,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;250MG
----
Product id = 21868
Application Number = 78728
Date of Application = 23,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21869
Application Number = 78728
Date of Application = 23,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;500MG
----
Product id = 21879
Application Number = 78905
Date of Application = 31,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;250MG
----
Product id = 21880
Application Number = 78905
Date of Application = 31,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21881
Application Number = 78905
Date of Application = 31,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;500MG
----