guanfacine-hydrochloride
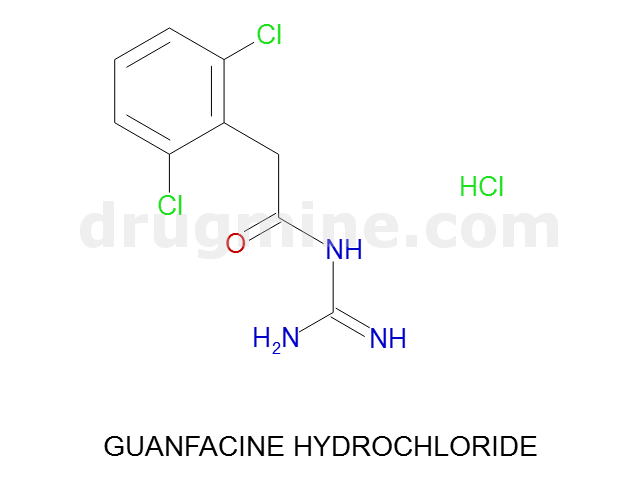


Name: GUANFACINE HYDROCHLORIDE
ID :
MW: 246
Number of atoms: 15
Molecular_Formula: C9H9Cl2N3O
Alogp: 2.03
Indication class : Antihypertensive
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
guanfacine hydrochloride containing products summary
There are in total 21 different products containing the active ingredient guanfacine hydrochloride. From the 21 drug products, 3 have been discontinued.Product id = 28550
Application Number = 19032
Date of Application = 27,, Oct, 1986
RX/OTC/DISCN = RX
Tradename = TENEX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PROMIUS PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 28551
Application Number = 19032
Date of Application = 7,, Nov, 1988
RX/OTC/DISCN = RX
Tradename = TENEX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PROMIUS PHARMA
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = EQ 2MG BASE
----
Product id = 28552
Application Number = 19032
Date of Application = 7,, Nov, 1988
RX/OTC/DISCN = DISCN
Tradename = TENEX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PROMIUS PHARMA
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 3MG BASE
----
Product id = 15998
Application Number = 22037
Date of Application = 2,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = INTUNIV
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SHIRE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 15999
Application Number = 22037
Date of Application = 2,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = INTUNIV
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SHIRE
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 16000
Application Number = 22037
Date of Application = 2,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = INTUNIV
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SHIRE
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 3MG BASE
----
Product id = 16001
Application Number = 22037
Date of Application = 2,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = INTUNIV
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SHIRE
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = EQ 4MG BASE
----
Product id = 22038
Application Number = 74145
Date of Application = 17,, Oct, 1995
RX/OTC/DISCN = RX
Tradename = GUANFACINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 22040
Application Number = 74145
Date of Application = 17,, Oct, 1995
RX/OTC/DISCN = RX
Tradename = GUANFACINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 22034
Application Number = 74673
Date of Application = 28,, Feb, 1997
RX/OTC/DISCN = RX
Tradename = GUANFACINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EPIC PHARMA LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 22035
Application Number = 74673
Date of Application = 28,, Feb, 1997
RX/OTC/DISCN = RX
Tradename = GUANFACINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EPIC PHARMA LLC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 22039
Application Number = 74762
Date of Application = 25,, Jun, 1997
RX/OTC/DISCN = DISCN
Tradename = GUANFACINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 22041
Application Number = 74762
Date of Application = 25,, Jun, 1997
RX/OTC/DISCN = DISCN
Tradename = GUANFACINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 22036
Application Number = 74796
Date of Application = 27,, Jan, 1997
RX/OTC/DISCN = RX
Tradename = GUANFACINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 22037
Application Number = 74796
Date of Application = 27,, Jan, 1997
RX/OTC/DISCN = RX
Tradename = GUANFACINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 22032
Application Number = 75109
Date of Application = 25,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = GUANFACINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 22033
Application Number = 75109
Date of Application = 25,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = GUANFACINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 15979
Application Number = 200881
Date of Application = 5,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = GUANFACINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 15980
Application Number = 200881
Date of Application = 5,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = GUANFACINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 15981
Application Number = 200881
Date of Application = 5,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = GUANFACINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 3MG BASE
----
Product id = 15982
Application Number = 200881
Date of Application = 5,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = GUANFACINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----