hydrocortisone-acetate
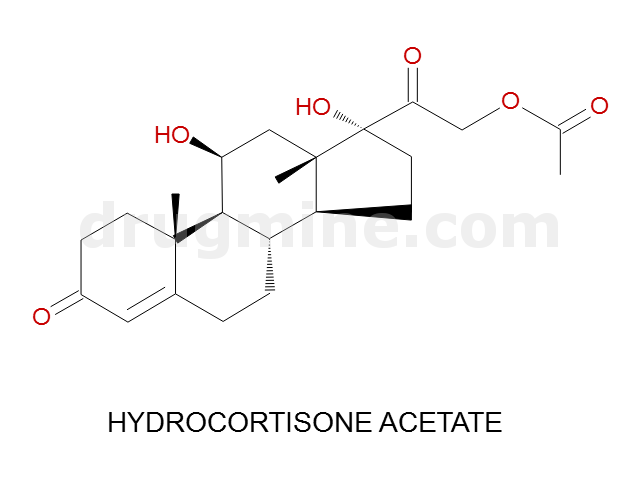
Name: HYDROCORTISONE ACETATE
ID :
MW: 404
Number of atoms: 29
Molecular_Formula: C23H32O6
Alogp: 1.662
Indication class : Glucocorticoid
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
hydrocortisone acetate containing products summary
There are in total 55 different products containing the active ingredient hydrocortisone acetate. From the 55 drug products, 40 have been discontinued.Product id = 8296
Application Number = 8228
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTONE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 8297
Application Number = 8228
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTONE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MERCK
ProductNo = 004
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 12434
Application Number = 8917
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTEF ACETATE
Route/format = TOPICAL / OINTMENT
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5% **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 12433
Application Number = 8917
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTEF ACETATE
Route/format = TOPICAL / OINTMENT
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 002
Tecode =
Rld = No
Strength = 1%
----
Product id = 12381
Application Number = 9018
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTONE
Route/format = OPHTHALMIC, OTIC / OINTMENT
Application Type = N
Applicant Name = MERCK
ProductNo = 003
Tecode =
Rld = No
Strength = 1.5%
----
Product id = 6858
Application Number = 9164
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTRIL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 6847
Application Number = 9378
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTEF ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 8273
Application Number = 9637
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTISONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = AKORN
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 8274
Application Number = 9637
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTISONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = AKORN
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 90
Application Number = 17351
Date of Application = 10,, Feb, 1982
RX/OTC/DISCN = RX
Tradename = CORTIFOAM
Route/format = RECTAL / AEROSOL, METERED
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 10%
----
Product id = 4256
Application Number = 40259
Date of Application = 29,, Jul, 1999
RX/OTC/DISCN = RX
Tradename = HYDROCORTISONE ACETATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FERNDALE LABS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2.5%
----
Product id = 4310
Application Number = 40396
Date of Application = 27,, Feb, 2001
RX/OTC/DISCN = RX
Tradename = MICORT-HC
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = SEBELA IRELAND LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5%
----
Product id = 4309
Application Number = 40398
Date of Application = 29,, Mar, 2002
RX/OTC/DISCN = RX
Tradename = MICORT-HC
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = SEBELA IRELAND LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 2%
----
Product id = 12365
Application Number = 50201
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = OPHTHOCORT
Route/format = OPHTHALMIC / OINTMENT
Application Type = N
Applicant Name = PARKEDALE
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG/GM;5MG/GM;10,000 UNITS/GM
----
Product id = 4987
Application Number = 50202
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLOROMYCETIN HYDROCORTISONE
Route/format = OPHTHALMIC / FOR SUSPENSION
Application Type = N
Applicant Name = PARKEDALE
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG/VIAL;25MG/VIAL
----
Product id = 4105
Application Number = 50218
Date of Application = 9,, Aug, 1985
RX/OTC/DISCN = RX
Tradename = CORTISPORIN
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = MONARCH PHARMS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.5%;EQ 3.5MG BASE/GM;10,000 UNITS/GM
----
Product id = 14596
Application Number = 50356
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = COLY-MYCIN S
Route/format = OTIC / SUSPENSION/DROPS
Application Type = N
Applicant Name = PAR STERILE PRODUCTS
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 3MG BASE/ML;10MG/ML;EQ 3.3MG BASE/ML;0.5MG/ML
----
Product id = 14544
Application Number = 60188
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = COR-OTICIN
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode =
Rld = No
Strength = 1.5%;EQ 3.5MG BASE/ML
----
Product id = 12348
Application Number = 60610
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NEO-CORTEF
Route/format = OPHTHALMIC / OINTMENT
Application Type = A
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%;EQ 3.5MG BASE/GM
----
Product id = 12349
Application Number = 60610
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NEO-CORTEF
Route/format = OPHTHALMIC / OINTMENT
Application Type = A
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 002
Tecode =
Rld = No
Strength = 1.5%;EQ 3.5MG BASE/GM
----
Product id = 14565
Application Number = 60612
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NEO-CORTEF
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = A
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode =
Rld = No
Strength = 1.5%;EQ 3.5MG BASE/ML
----
Product id = 14564
Application Number = 60612
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NEO-CORTEF
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = A
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 002
Tecode =
Rld = No
Strength = 0.5%;EQ 3.5MG BASE/ML
----
Product id = 12309
Application Number = 60731
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BACITRACIN-NEOMYCIN-POLYMYXIN W/ HYDROCORTISONE ACETATE
Route/format = OPHTHALMIC / OINTMENT
Application Type = A
Applicant Name = ALTANA
ProductNo = 002
Tecode =
Rld = No
Strength = 400 UNITS/GM;1%;EQ 3.5MG BASE/GM;10,000 UNITS/GM
----
Product id = 12548
Application Number = 60751
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NEO-CORTEF
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%;EQ 3.5MG BASE/GM
----
Product id = 12549
Application Number = 60751
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NEO-CORTEF
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 002
Tecode =
Rld = No
Strength = 1%;EQ 3.5MG BASE/GM
----
Product id = 12550
Application Number = 60751
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NEO-CORTEF
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 003
Tecode =
Rld = No
Strength = 2.5%;EQ 3.5MG BASE/GM
----
Product id = 14631
Application Number = 61016
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TERRA-CORTRIL
Route/format = OPHTHALMIC / SUSPENSION
Application Type = A
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = No
Strength = 1.5%;EQ 5MG BASE/ML
----
Product id = 4331
Application Number = 61049
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NEO-CORTEF
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode =
Rld = No
Strength = 1%;EQ 3.5MG BASE/GM
----
Product id = 4332
Application Number = 61049
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NEO-CORTEF
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 002
Tecode =
Rld = No
Strength = 2.5%;EQ 3.5MG BASE/GM
----
Product id = 12310
Application Number = 62166
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = BACITRACIN-NEOMYCIN-POLYMYXIN W/ HYDROCORTISONE ACETATE
Route/format = OPHTHALMIC / OINTMENT
Application Type = A
Applicant Name = PERRIGO CO TENNESSEE
ProductNo = 002
Tecode =
Rld = Yes
Strength = 400 UNITS/GM;1%;EQ 3.5MG BASE/GM;10,000 UNITS/GM
----
Product id = 4255
Application Number = 80419
Date of Application = 25,, Jan, 1982
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTISONE ACETATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = CENCI
ProductNo = 001
Tecode =
Rld = No
Strength = 1%
----
Product id = 4076
Application Number = 80505
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CARMOL HC
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 1%;10%
----
Product id = 12341
Application Number = 80828
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTISONE ACETATE
Route/format = OPHTHALMIC / OINTMENT
Application Type = A
Applicant Name = FERA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 4216
Application Number = 81274
Date of Application = 19,, Jun, 1992
RX/OTC/DISCN = DISCN
Tradename = HEMSOL-HC
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ABLE
ProductNo = 001
Tecode =
Rld = No
Strength = 1%
----
Product id = 8277
Application Number = 83128
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTISONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 12627
Application Number = 83205
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ORABASE HCA
Route/format = TOPICAL / PASTE
Application Type = A
Applicant Name = COLGATE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 12253
Application Number = 83213
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PRAMOSONE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = FERNDALE LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.5%;1%
----
Product id = 8275
Application Number = 83739
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTISONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEL MAR
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 8276
Application Number = 83739
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTISONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEL MAR
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 8278
Application Number = 83759
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTISONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 8279
Application Number = 83759
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTISONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 4366
Application Number = 83778
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = PRAMOSONE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = SEBELA IRELAND LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%;1%
----
Product id = 8280
Application Number = 85214
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTISONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 4367
Application Number = 85368
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = PRAMOSONE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = SEBELA IRELAND LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 1%;1%
----
Product id = 12255
Application Number = 85979
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = PRAMOSONE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = SEBELA IRELAND LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5%;1%
----
Product id = 12254
Application Number = 85980
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = PRAMOSONE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = SEBELA IRELAND LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 1%;1%
----
Product id = 12655
Application Number = 85981
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = HYDROCORTISONE ACETATE
Route/format = FOR RX COMPOUNDING / POWDER
Application Type = A
Applicant Name = X GEN PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 100%
----
Product id = 4258
Application Number = 86050
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTISONE ACETATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 4259
Application Number = 86052
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTISONE ACETATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 1%
----
Product id = 95
Application Number = 86195
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = PROCTOFOAM HC
Route/format = TOPICAL / AEROSOL, METERED
Application Type = A
Applicant Name = MEDA PHARMS
ProductNo = 001
Tecode = BX
Rld = No
Strength = 1%;1%
----
Product id = 12192
Application Number = 86207
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DRICORT
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = INGRAM PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 92
Application Number = 86457
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = EPIFOAM
Route/format = TOPICAL / AEROSOL, METERED
Application Type = A
Applicant Name = MEDA PHARMS
ProductNo = 001
Tecode = BX
Rld = No
Strength = 1%;1%
----
Product id = 94
Application Number = 89440
Date of Application = 17,, May, 1988
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTISONE ACETATE 1% AND PRAMOXINE HYDROCHLORIDE 1%
Route/format = TOPICAL / AEROSOL, METERED
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 1%;1%
----
Product id = 4448
Application Number = 89472
Date of Application = 13,, Jun, 1988
RX/OTC/DISCN = RX
Tradename = U-CORT
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 1%;10%
----
Product id = 4257
Application Number = 89914
Date of Application = 3,, Jan, 1989
RX/OTC/DISCN = DISCN
Tradename = HYDROCORTISONE ACETATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 1%
----