ibuprofen
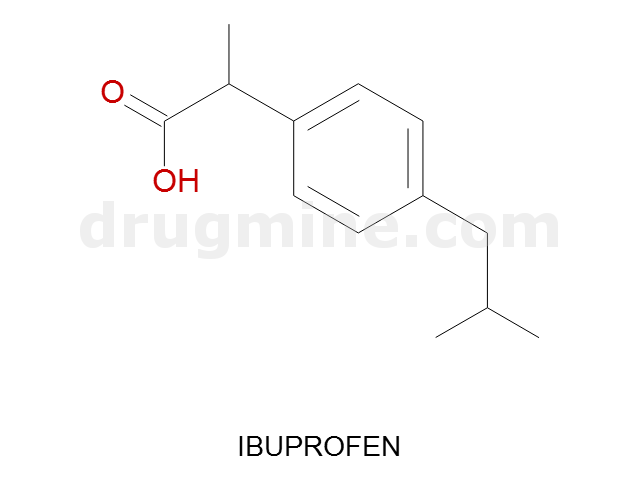

Name: IBUPROFEN
ID :
MW: 206
Number of atoms: 15
Molecular_Formula: C13H18O2
Alogp: 3.607
Indication class : Anti-Inflammatory
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
ibuprofen containing products summary
There are in total 246 different products containing the active ingredient ibuprofen. From the 246 drug products, 111 have been discontinued.Product id = 25093
Application Number = 17463
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MOTRIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MCNEIL CONSUMER
ProductNo = 002
Tecode =
Rld = No
Strength = 400MG
----
Product id = 25092
Application Number = 17463
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MOTRIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MCNEIL CONSUMER
ProductNo = 003
Tecode =
Rld = No
Strength = 300MG
----
Product id = 25094
Application Number = 17463
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MOTRIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MCNEIL CONSUMER
ProductNo = 004
Tecode =
Rld = No
Strength = 600MG
----
Product id = 25095
Application Number = 17463
Date of Application = 22,, May, 1985
RX/OTC/DISCN = DISCN
Tradename = MOTRIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MCNEIL CONSUMER
ProductNo = 005
Tecode =
Rld = No
Strength = 800MG
----
Product id = 22696
Application Number = 18197
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = IBU
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BASF
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 27812
Application Number = 18197
Date of Application = 5,, Mar, 1984
RX/OTC/DISCN = DISCN
Tradename = RUFEN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BASF
ProductNo = 002
Tecode =
Rld = No
Strength = 600MG
----
Product id = 17385
Application Number = 18989
Date of Application = 18,, May, 1984
RX/OTC/DISCN = OTC
Tradename = ADVIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 25513
Application Number = 19012
Date of Application = 18,, May, 1984
RX/OTC/DISCN = DISCN
Tradename = NUPRIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MCNEIL
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 25514
Application Number = 19012
Date of Application = 29,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = NUPRIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MCNEIL
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG
----
Product id = 25097
Application Number = 19012
Date of Application = 17,, Dec, 1990
RX/OTC/DISCN = OTC
Tradename = MOTRIN IB
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MCNEIL
ProductNo = 003
Tecode =
Rld = Yes
Strength = 200MG
----
Product id = 25098
Application Number = 19012
Date of Application = 25,, Feb, 2000
RX/OTC/DISCN = OTC
Tradename = MOTRIN MIGRAINE PAIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MCNEIL
ProductNo = 004
Tecode =
Rld = Yes
Strength = 200MG
----
Product id = 17389
Application Number = 19771
Date of Application = 19,, Sep, 1989
RX/OTC/DISCN = OTC
Tradename = ADVIL COLD AND SINUS
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = 200MG;30MG
----
Product id = 14712
Application Number = 19784
Date of Application = 18,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = IBU
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/5ML
----
Product id = 14654
Application Number = 19833
Date of Application = 19,, Sep, 1989
RX/OTC/DISCN = DISCN
Tradename = CHILDREN'S ADVIL
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = WYETH CONS
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG/5ML
----
Product id = 14745
Application Number = 19842
Date of Application = 19,, Sep, 1989
RX/OTC/DISCN = DISCN
Tradename = MOTRIN
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = MCNEIL CONSUMER
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/5ML
----
Product id = 28070
Application Number = 19899
Date of Application = 31,, Dec, 1992
RX/OTC/DISCN = OTC
Tradename = SINE-AID IB
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MCNEIL CONS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG;30MG
----
Product id = 15339
Application Number = 20135
Date of Application = 16,, Nov, 1994
RX/OTC/DISCN = DISCN
Tradename = MOTRIN
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = MCNEIL PED
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 15340
Application Number = 20135
Date of Application = 16,, Nov, 1994
RX/OTC/DISCN = DISCN
Tradename = MOTRIN
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = MCNEIL PED
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 23129
Application Number = 20267
Date of Application = 13,, Dec, 1996
RX/OTC/DISCN = OTC
Tradename = JUNIOR STRENGTH ADVIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 939
Application Number = 20402
Date of Application = 20,, Apr, 1995
RX/OTC/DISCN = OTC
Tradename = ADVIL LIQUI-GELS
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 200MG FREE ACID AND POTASSIUM SALT
----
Product id = 940
Application Number = 20402
Date of Application = 16,, Mar, 2000
RX/OTC/DISCN = OTC
Tradename = ADVIL MIGRAINE LIQUI-GELS
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 200MG FREE ACID AND POTASSIUM SALT
----
Product id = 25096
Application Number = 20418
Date of Application = 16,, Nov, 1994
RX/OTC/DISCN = DISCN
Tradename = MOTRIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MCNEIL PED
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 14591
Application Number = 20476
Date of Application = 25,, May, 1995
RX/OTC/DISCN = DISCN
Tradename = MOTRIN
Route/format = ORAL / SUSPENSION/DROPS
Application Type = N
Applicant Name = MCNEIL
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG/ML
----
Product id = 14664
Application Number = 20516
Date of Application = 16,, Jun, 1995
RX/OTC/DISCN = OTC
Tradename = CHILDREN'S MOTRIN
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = MCNEIL
ProductNo = 001
Tecode =
Rld = Yes
Strength = 100MG/5ML
----
Product id = 14653
Application Number = 20589
Date of Application = 27,, Jun, 1996
RX/OTC/DISCN = OTC
Tradename = CHILDREN'S ADVIL
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/5ML
----
Product id = 14657
Application Number = 20589
Date of Application = 7,, Nov, 1997
RX/OTC/DISCN = OTC
Tradename = CHILDREN'S ADVIL-FLAVORED
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG/5ML
----
Product id = 15242
Application Number = 20601
Date of Application = 15,, Nov, 1996
RX/OTC/DISCN = OTC
Tradename = CHILDREN'S MOTRIN
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = MCNEIL CONS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 15272
Application Number = 20601
Date of Application = 15,, Nov, 1996
RX/OTC/DISCN = OTC
Tradename = JUNIOR STRENGTH MOTRIN
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = MCNEIL CONS
ProductNo = 003
Tecode =
Rld = Yes
Strength = 100MG
----
Product id = 23131
Application Number = 20602
Date of Application = 10,, Jun, 1996
RX/OTC/DISCN = OTC
Tradename = JUNIOR STRENGTH MOTRIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MCNEIL CONS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 14587
Application Number = 20603
Date of Application = 10,, Jun, 1996
RX/OTC/DISCN = OTC
Tradename = CHILDREN'S MOTRIN
Route/format = ORAL / SUSPENSION/DROPS
Application Type = N
Applicant Name = MCNEIL CONS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 40MG/ML
----
Product id = 29580
Application Number = 20716
Date of Application = 23,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = VICOPROFEN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBVIE
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 7.5MG;200MG
----
Product id = 14593
Application Number = 20812
Date of Application = 30,, Jan, 1998
RX/OTC/DISCN = OTC
Tradename = PEDIATRIC ADVIL
Route/format = ORAL / SUSPENSION/DROPS
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = 100MG/2.5ML
----
Product id = 15230
Application Number = 20944
Date of Application = 18,, Dec, 1998
RX/OTC/DISCN = OTC
Tradename = CHILDREN'S ADVIL
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 15271
Application Number = 20944
Date of Application = 18,, Dec, 1998
RX/OTC/DISCN = OTC
Tradename = JUNIOR STRENGTH ADVIL
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 14665
Application Number = 21128
Date of Application = 1,, Aug, 2000
RX/OTC/DISCN = OTC
Tradename = CHILDREN'S MOTRIN COLD
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = MCNEIL CONS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 100MG/5ML;15MG/5ML
----
Product id = 14656
Application Number = 21373
Date of Application = 18,, Apr, 2002
RX/OTC/DISCN = OTC
Tradename = CHILDREN'S ADVIL COLD
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/5ML;15MG/5ML
----
Product id = 938
Application Number = 21374
Date of Application = 30,, May, 2002
RX/OTC/DISCN = OTC
Tradename = ADVIL COLD AND SINUS
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 200MG FREE ACID AND POTASSIUM SALT;30MG
----
Product id = 19971
Application Number = 21378
Date of Application = 26,, Nov, 2004
RX/OTC/DISCN = DISCN
Tradename = COMBUNOX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = FOREST LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG;5MG
----
Product id = 941
Application Number = 21393
Date of Application = 21,, Dec, 2005
RX/OTC/DISCN = OTC
Tradename = ADVIL PM
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = 25MG;EQ 200MG FREE ACID AND POTASSIUM SALT
----
Product id = 17391
Application Number = 21394
Date of Application = 21,, Dec, 2005
RX/OTC/DISCN = OTC
Tradename = ADVIL PM
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = 38MG;200MG
----
Product id = 17388
Application Number = 21441
Date of Application = 19,, Dec, 2002
RX/OTC/DISCN = OTC
Tradename = ADVIL ALLERGY SINUS
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2MG;200MG;30MG
----
Product id = 2507
Application Number = 21472
Date of Application = 18,, Oct, 2002
RX/OTC/DISCN = OTC
Tradename = MIDOL LIQUID GELS
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BANNER LIFE SCIENCES
ProductNo = 001
Tecode =
Rld = Yes
Strength = 200MG
----
Product id = 14655
Application Number = 21587
Date of Application = 24,, Feb, 2004
RX/OTC/DISCN = OTC
Tradename = CHILDREN'S ADVIL ALLERGY SINUS
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = 1MG/5ML;100MG/5ML;15MG/5ML
----
Product id = 14660
Application Number = 21604
Date of Application = 7,, Jan, 2004
RX/OTC/DISCN = OTC
Tradename = CHILDREN'S ELIXSURE
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = MOBERG PHARMA NORTH
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/5ML
----
Product id = 17387
Application Number = 22113
Date of Application = 21,, Dec, 2011
RX/OTC/DISCN = OTC
Tradename = ADVIL ALLERGY AND CONGESTION RELIEF
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = 4MG;200MG;10MG
----
Product id = 13545
Application Number = 22348
Date of Application = 11,, Jun, 2009
RX/OTC/DISCN = DISCN
Tradename = CALDOLOR
Route/format = INTRAVENOUS / SOLUTION
Application Type = N
Applicant Name = CUMBERLAND PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG/4ML (100MG/ML)
----
Product id = 13546
Application Number = 22348
Date of Application = 11,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = CALDOLOR
Route/format = INTRAVENOUS / SOLUTION
Application Type = N
Applicant Name = CUMBERLAND PHARMS
ProductNo = 002
Tecode =
Rld = Yes
Strength = 800MG/8ML (100MG/ML)
----
Product id = 20762
Application Number = 22519
Date of Application = 23,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = DUEXIS
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = HORIZON PHARMA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 26.6MG;800MG
----
Product id = 17390
Application Number = 22565
Date of Application = 27,, May, 2010
RX/OTC/DISCN = OTC
Tradename = ADVIL CONGESTION RELIEF
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = 200MG;10MG
----
Product id = 22832
Application Number = 70038
Date of Application = 6,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22834
Application Number = 70041
Date of Application = 6,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22769
Application Number = 70045
Date of Application = 24,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22770
Application Number = 70057
Date of Application = 24,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22765
Application Number = 70079
Date of Application = 24,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22766
Application Number = 70080
Date of Application = 24,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22759
Application Number = 70081
Date of Application = 16,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MCNEIL
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22695
Application Number = 70083
Date of Application = 22,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = IBU
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BASF
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22697
Application Number = 70088
Date of Application = 8,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = IBU
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BASF
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22698
Application Number = 70099
Date of Application = 29,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = IBU
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BASF
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22779
Application Number = 70328
Date of Application = 6,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 22780
Application Number = 70329
Date of Application = 6,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22781
Application Number = 70330
Date of Application = 6,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22828
Application Number = 70435
Date of Application = 5,, Mar, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22833
Application Number = 70436
Date of Application = 21,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22835
Application Number = 70437
Date of Application = 21,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22844
Application Number = 70469
Date of Application = 29,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = IBUPROHM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = OHM LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 24174
Application Number = 70475
Date of Application = 6,, Feb, 1986
RX/OTC/DISCN = DISCN
Tradename = MEDIPREN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MCNEIL
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22760
Application Number = 70476
Date of Application = 16,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MCNEIL
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22777
Application Number = 70481
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22762
Application Number = 70493
Date of Application = 24,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22705
Application Number = 70556
Date of Application = 14,, Jun, 1985
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 24931
Application Number = 70591
Date of Application = 2,, Sep, 1987
RX/OTC/DISCN = DISCN
Tradename = MIDOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BAYER
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 2505
Application Number = 70626
Date of Application = 2,, Sep, 1987
RX/OTC/DISCN = DISCN
Tradename = MIDOL
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = BAYER
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 22747
Application Number = 70629
Date of Application = 19,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22748
Application Number = 70630
Date of Application = 19,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22813
Application Number = 70709
Date of Application = 25,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22799
Application Number = 70733
Date of Application = 19,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22803
Application Number = 70734
Date of Application = 12,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 22804
Application Number = 70735
Date of Application = 12,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22806
Application Number = 70736
Date of Application = 12,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22699
Application Number = 70745
Date of Application = 23,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = IBU
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BASF
ProductNo = 001
Tecode =
Rld = No
Strength = 800MG
----
Product id = 22776
Application Number = 70818
Date of Application = 26,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = OHM LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22763
Application Number = 70908
Date of Application = 26,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22761
Application Number = 70985
Date of Application = 2,, Oct, 1987
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MERRO PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22782
Application Number = 70986
Date of Application = 25,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 800MG
----
Product id = 24932
Application Number = 71001
Date of Application = 2,, Sep, 1987
RX/OTC/DISCN = DISCN
Tradename = MIDOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BAYER
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 2506
Application Number = 71002
Date of Application = 2,, Sep, 1987
RX/OTC/DISCN = DISCN
Tradename = MIDOL
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = BAYER
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 22734
Application Number = 71027
Date of Application = 29,, Sep, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22735
Application Number = 71028
Date of Application = 23,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 22736
Application Number = 71029
Date of Application = 23,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22737
Application Number = 71030
Date of Application = 23,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22703
Application Number = 71057
Date of Application = 11,, Aug, 1988
RX/OTC/DISCN = OTC
Tradename = IBU-TAB 200
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALRA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22700
Application Number = 71058
Date of Application = 11,, Aug, 1988
RX/OTC/DISCN = RX
Tradename = IBU-TAB
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALRA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 22701
Application Number = 71059
Date of Application = 11,, Aug, 1988
RX/OTC/DISCN = RX
Tradename = IBU-TAB
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALRA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 600MG
----
Product id = 17318
Application Number = 71065
Date of Application = 28,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = ACHES-N-PAIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22793
Application Number = 71122
Date of Application = 3,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22795
Application Number = 71123
Date of Application = 19,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 22796
Application Number = 71124
Date of Application = 19,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22797
Application Number = 71125
Date of Application = 19,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22739
Application Number = 71144
Date of Application = 20,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22744
Application Number = 71145
Date of Application = 23,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22745
Application Number = 71146
Date of Application = 23,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22740
Application Number = 71154
Date of Application = 27,, Oct, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22775
Application Number = 71163
Date of Application = 15,, Jul, 1986
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = OHM
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22843
Application Number = 71214
Date of Application = 1,, Dec, 1986
RX/OTC/DISCN = OTC
Tradename = IBUPROHM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = OHM LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 24175
Application Number = 71215
Date of Application = 26,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = MEDIPREN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MCNEIL
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22820
Application Number = 71229
Date of Application = 1,, Apr, 1987
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22823
Application Number = 71230
Date of Application = 22,, Oct, 1986
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 22824
Application Number = 71231
Date of Application = 22,, Oct, 1986
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 22826
Application Number = 71232
Date of Application = 22,, Oct, 1986
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 600MG
----
Product id = 22706
Application Number = 71264
Date of Application = 25,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 800MG
----
Product id = 26734
Application Number = 71265
Date of Application = 15,, Oct, 1986
RX/OTC/DISCN = OTC
Tradename = PROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CONTRACT PHARMACAL
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22749
Application Number = 71266
Date of Application = 15,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEINER
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 22722
Application Number = 71267
Date of Application = 15,, Oct, 1986
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CONTRACT PHARMACAL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 22723
Application Number = 71268
Date of Application = 15,, Oct, 1986
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CONTRACT PHARMACAL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 600MG
----
Product id = 22708
Application Number = 71333
Date of Application = 17,, Feb, 1987
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22710
Application Number = 71334
Date of Application = 25,, Nov, 1986
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 22712
Application Number = 71335
Date of Application = 25,, Nov, 1986
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 600MG
----
Product id = 22831
Application Number = 71338
Date of Application = 1,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 22767
Application Number = 71448
Date of Application = 18,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 800MG
----
Product id = 22764
Application Number = 71462
Date of Application = 2,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22836
Application Number = 71547
Date of Application = 2,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 800MG
----
Product id = 22778
Application Number = 71575
Date of Application = 8,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22821
Application Number = 71639
Date of Application = 2,, Feb, 1988
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22825
Application Number = 71644
Date of Application = 1,, Feb, 1988
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 22794
Application Number = 71664
Date of Application = 3,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22790
Application Number = 71666
Date of Application = 18,, Jun, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22791
Application Number = 71667
Date of Application = 18,, Jun, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22792
Application Number = 71668
Date of Application = 18,, Jun, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode =
Rld = No
Strength = 800MG
----
Product id = 22717
Application Number = 71732
Date of Application = 10,, Sep, 1987
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CONTRACT PHARMACAL
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22718
Application Number = 71735
Date of Application = 10,, Sep, 1987
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CONTRACT PHARMACAL
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22829
Application Number = 71765
Date of Application = 4,, Sep, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22746
Application Number = 71769
Date of Application = 8,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 800MG
----
Product id = 22704
Application Number = 71773
Date of Application = 16,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22800
Application Number = 71807
Date of Application = 25,, Feb, 1988
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22768
Application Number = 71870
Date of Application = 5,, May, 1988
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22830
Application Number = 71905
Date of Application = 8,, Mar, 1988
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22837
Application Number = 71911
Date of Application = 13,, Oct, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 800MG
----
Product id = 22714
Application Number = 71935
Date of Application = 13,, Oct, 1987
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 22808
Application Number = 71938
Date of Application = 14,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 800MG
----
Product id = 22798
Application Number = 71964
Date of Application = 1,, Feb, 1988
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 800MG
----
Product id = 22702
Application Number = 71965
Date of Application = 11,, Aug, 1988
RX/OTC/DISCN = DISCN
Tradename = IBU-TAB
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALRA
ProductNo = 001
Tecode =
Rld = No
Strength = 800MG
----
Product id = 22771
Application Number = 71999
Date of Application = 3,, Dec, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 800MG
----
Product id = 22827
Application Number = 72004
Date of Application = 18,, Nov, 1987
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 25511
Application Number = 72035
Date of Application = 16,, Feb, 1988
RX/OTC/DISCN = DISCN
Tradename = NUPRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BRISTOL MYERS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 25512
Application Number = 72036
Date of Application = 16,, Feb, 1988
RX/OTC/DISCN = DISCN
Tradename = NUPRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BRISTOL MYERS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22741
Application Number = 72040
Date of Application = 29,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22805
Application Number = 72064
Date of Application = 14,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22807
Application Number = 72065
Date of Application = 14,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 28435
Application Number = 72095
Date of Application = 8,, Dec, 1987
RX/OTC/DISCN = OTC
Tradename = TAB-PROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22783
Application Number = 72096
Date of Application = 8,, Dec, 1987
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 18823
Application Number = 72097
Date of Application = 8,, Dec, 1987
RX/OTC/DISCN = DISCN
Tradename = CAP-PROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22784
Application Number = 72098
Date of Application = 8,, Dec, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22738
Application Number = 72137
Date of Application = 5,, Feb, 1988
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 001
Tecode =
Rld = No
Strength = 800MG
----
Product id = 22809
Application Number = 72169
Date of Application = 11,, Dec, 1987
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 800MG
----
Product id = 22709
Application Number = 72199
Date of Application = 23,, May, 1988
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22822
Application Number = 72249
Date of Application = 10,, Jan, 1989
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22719
Application Number = 72299
Date of Application = 1,, Jul, 1988
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CONTRACT PHARMACAL
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22724
Application Number = 72300
Date of Application = 1,, Jul, 1988
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CONTRACT PHARMACAL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 22742
Application Number = 72901
Date of Application = 19,, Dec, 1991
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22743
Application Number = 72903
Date of Application = 19,, Dec, 1991
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22758
Application Number = 73019
Date of Application = 30,, Mar, 1994
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MCNEIL
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22816
Application Number = 73141
Date of Application = 29,, May, 1992
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22817
Application Number = 73343
Date of Application = 30,, Jun, 1992
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22818
Application Number = 73344
Date of Application = 30,, Jun, 1992
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22819
Application Number = 73345
Date of Application = 30,, Jun, 1992
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 800MG
----
Product id = 22720
Application Number = 73691
Date of Application = 25,, Feb, 1994
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CONTRACT PHARMACAL
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22801
Application Number = 74525
Date of Application = 15,, Dec, 1995
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22802
Application Number = 74533
Date of Application = 15,, Dec, 1995
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22845
Application Number = 74567
Date of Application = 17,, Apr, 2001
RX/OTC/DISCN = OTC
Tradename = IBUPROHM COLD AND SINUS
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = OHM LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG;30MG
----
Product id = 2223
Application Number = 74782
Date of Application = 6,, Jul, 1998
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = CONTRACT PHARMACAL
ProductNo = 001
Tecode =
Rld = Yes
Strength = 200MG
----
Product id = 14713
Application Number = 74916
Date of Application = 30,, Apr, 1999
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/5ML
----
Product id = 22721
Application Number = 74931
Date of Application = 20,, Jul, 1998
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CONTRACT PHARMACAL
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 14663
Application Number = 74937
Date of Application = 22,, Dec, 1998
RX/OTC/DISCN = OTC
Tradename = CHILDREN'S IBUPROFEN
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/5ML
----
Product id = 14714
Application Number = 74978
Date of Application = 25,, Mar, 1998
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 100MG/5ML
----
Product id = 22751
Application Number = 75010
Date of Application = 1,, Mar, 1999
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LNK
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22752
Application Number = 75139
Date of Application = 1,, Mar, 1999
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LNK
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 14588
Application Number = 75217
Date of Application = 16,, Dec, 1998
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / SUSPENSION/DROPS
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG/ML
----
Product id = 23130
Application Number = 75367
Date of Application = 22,, Apr, 1999
RX/OTC/DISCN = OTC
Tradename = JUNIOR STRENGTH IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 22841
Application Number = 75588
Date of Application = 8,, Apr, 2002
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CONTRACT PHARMACAL
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG;30MG
----
Product id = 22725
Application Number = 75661
Date of Application = 12,, Dec, 2001
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22726
Application Number = 75682
Date of Application = 14,, Nov, 2001
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 22727
Application Number = 75682
Date of Application = 14,, Nov, 2001
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 600MG
----
Product id = 22728
Application Number = 75682
Date of Application = 14,, Nov, 2001
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LA
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 800MG
----
Product id = 22785
Application Number = 75995
Date of Application = 14,, Mar, 2002
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22516
Application Number = 76023
Date of Application = 11,, Apr, 2003
RX/OTC/DISCN = RX
Tradename = HYDROCODONE BITARTRATE AND IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 7.5MG;200MG
----
Product id = 22730
Application Number = 76112
Date of Application = 31,, Oct, 2001
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 22731
Application Number = 76112
Date of Application = 31,, Oct, 2001
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 600MG
----
Product id = 22732
Application Number = 76112
Date of Application = 31,, Oct, 2001
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 22729
Application Number = 76117
Date of Application = 20,, Nov, 2001
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 15263
Application Number = 76359
Date of Application = 16,, Jan, 2004
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 15264
Application Number = 76359
Date of Application = 16,, Jan, 2004
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = PERRIGO
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 22716
Application Number = 76460
Date of Application = 26,, Nov, 2003
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AVEMA PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 14717
Application Number = 76478
Date of Application = 5,, Nov, 2003
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/5ML;15MG/5ML
----
Product id = 22509
Application Number = 76604
Date of Application = 31,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = HYDROCODONE BITARTRATE AND IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 7.5MG;200MG
----
Product id = 22511
Application Number = 76642
Date of Application = 12,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = HYDROCODONE BITARTRATE AND IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 7.5MG;200MG
----
Product id = 22510
Application Number = 76642
Date of Application = 18,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = HYDROCODONE BITARTRATE AND IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG;200MG
----
Product id = 27437
Application Number = 76642
Date of Application = 19,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = REPREXAIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2.5MG;200MG
----
Product id = 27438
Application Number = 76642
Date of Application = 19,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = REPREXAIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 004
Tecode = AB
Rld = No
Strength = 10MG;200MG
----
Product id = 22750
Application Number = 76741
Date of Application = 17,, Jun, 2004
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LNK
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 14716
Application Number = 76925
Date of Application = 23,, Sep, 2004
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = PERRIGO R AND D
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG/5ML
----
Product id = 22787
Application Number = 77114
Date of Application = 18,, Jul, 2005
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO R AND D
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 22788
Application Number = 77114
Date of Application = 18,, Jul, 2005
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO R AND D
ProductNo = 002
Tecode = AB
Rld = No
Strength = 600MG
----
Product id = 22789
Application Number = 77114
Date of Application = 18,, Jul, 2005
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO R AND D
ProductNo = 003
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 2225
Application Number = 77338
Date of Application = 10,, Jul, 2009
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = P AND L DEV LLC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 200MG FREE ACID AND POTASSIUM SALT
----
Product id = 22786
Application Number = 77349
Date of Application = 21,, Jun, 2005
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO R AND D
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22508
Application Number = 77454
Date of Application = 23,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = HYDROCODONE BITARTRATE AND IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;200MG
----
Product id = 22842
Application Number = 77628
Date of Application = 14,, Aug, 2006
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG;30MG
----
Product id = 22518
Application Number = 77723
Date of Application = 6,, Nov, 2006
RX/OTC/DISCN = RX
Tradename = HYDROCODONE BITARTRATE AND IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 7.5MG;200MG
----
Product id = 22519
Application Number = 77723
Date of Application = 6,, Nov, 2006
RX/OTC/DISCN = RX
Tradename = HYDROCODONE BITARTRATE AND IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;200MG
----
Product id = 22517
Application Number = 77727
Date of Application = 6,, Nov, 2006
RX/OTC/DISCN = RX
Tradename = HYDROCODONE BITARTRATE AND IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;200MG
----
Product id = 22772
Application Number = 78132
Date of Application = 10,, Sep, 2007
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NORTHSTAR HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 22773
Application Number = 78132
Date of Application = 10,, Sep, 2007
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NORTHSTAR HLTHCARE
ProductNo = 002
Tecode =
Rld = No
Strength = 600MG
----
Product id = 22774
Application Number = 78132
Date of Application = 10,, Sep, 2007
RX/OTC/DISCN = DISCN
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NORTHSTAR HLTHCARE
ProductNo = 003
Tecode =
Rld = No
Strength = 800MG
----
Product id = 25860
Application Number = 78316
Date of Application = 29,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = OXYCODONE HYDROCHLORIDE AND IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR LABS INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 400MG;5MG
----
Product id = 22810
Application Number = 78329
Date of Application = 5,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SHASUN USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 22811
Application Number = 78329
Date of Application = 5,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SHASUN USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 600MG
----
Product id = 22812
Application Number = 78329
Date of Application = 5,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SHASUN USA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 25861
Application Number = 78394
Date of Application = 26,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = OXYCODONE HYDROCHLORIDE AND IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG;5MG
----
Product id = 22711
Application Number = 78558
Date of Application = 18,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 22713
Application Number = 78558
Date of Application = 18,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 600MG
----
Product id = 22715
Application Number = 78558
Date of Application = 18,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 2222
Application Number = 78682
Date of Application = 24,, Mar, 2009
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = BANNER LIFE SCIENCES
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 200MG FREE ACID AND POTASSIUM SALT
----
Product id = 25859
Application Number = 78769
Date of Application = 4,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = OXYCODONE HYDROCHLORIDE AND IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG;5MG
----
Product id = 14589
Application Number = 79058
Date of Application = 31,, Aug, 2009
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / SUSPENSION/DROPS
Application Type = A
Applicant Name = TRIS PHARMA INC
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG/ML
----
Product id = 22839
Application Number = 79113
Date of Application = 22,, Dec, 2008
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN AND DIPHENHYDRAMINE CITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO R AND D
ProductNo = 001
Tecode =
Rld = No
Strength = 38MG;200MG
----
Product id = 22814
Application Number = 79129
Date of Application = 28,, Mar, 2011
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SVADS HOLDINGS SA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22733
Application Number = 79174
Date of Application = 10,, Dec, 2010
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GRANULES INDIA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 2224
Application Number = 79205
Date of Application = 26,, Jun, 2009
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MARKSANS PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 200MG FREE ACID AND POTASSIUM SALT
----
Product id = 22707
Application Number = 79233
Date of Application = 18,, Mar, 2014
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 2226
Application Number = 90397
Date of Application = 22,, Nov, 2010
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN AND DIPHENHYDRAMINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = BANNER LIFE SCIENCES
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;EQ 200MG FREE ACID AND POTASSIUM SALT
----
Product id = 22838
Application Number = 90619
Date of Application = 8,, Jul, 2009
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN AND DIPHENHYDRAMINE CITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 38MG;200MG
----
Product id = 22755
Application Number = 90796
Date of Application = 21,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MARKSANS PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 22756
Application Number = 90796
Date of Application = 21,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MARKSANS PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 600MG
----
Product id = 22757
Application Number = 90796
Date of Application = 21,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MARKSANS PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 22753
Application Number = 91237
Date of Application = 8,, Feb, 2011
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MARKSANS PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22754
Application Number = 91239
Date of Application = 1,, Feb, 2011
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MARKSANS PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22815
Application Number = 91355
Date of Application = 4,, Apr, 2011
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SVADS HOLDINGS SA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22512
Application Number = 91633
Date of Application = 28,, May, 2013
RX/OTC/DISCN = RX
Tradename = HYDROCODONE BITARTRATE AND IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;200MG
----
Product id = 22513
Application Number = 91633
Date of Application = 28,, May, 2013
RX/OTC/DISCN = RX
Tradename = HYDROCODONE BITARTRATE AND IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG;200MG
----
Product id = 22514
Application Number = 91633
Date of Application = 28,, May, 2013
RX/OTC/DISCN = RX
Tradename = HYDROCODONE BITARTRATE AND IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 7.5MG;200MG
----
Product id = 22515
Application Number = 91633
Date of Application = 28,, May, 2013
RX/OTC/DISCN = RX
Tradename = HYDROCODONE BITARTRATE AND IBUPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 10MG;200MG
----
Product id = 14715
Application Number = 200457
Date of Application = 18,, Aug, 2011
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/5ML
----
Product id = 2227
Application Number = 200888
Date of Application = 5,, Mar, 2012
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN AND DIPHENHYDRAMINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = STRIDES PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;EQ 200MG FREE ACID AND POTASSIUM SALT
----
Product id = 2221
Application Number = 202300
Date of Application = 23,, Dec, 2011
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 200MG FREE ACID AND POTASSIUM SALT
----
Product id = 22840
Application Number = 203200
Date of Application = 3,, Jul, 2014
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN AND PHENYLEPHRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO R AND D
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG;10MG
----