iopromide
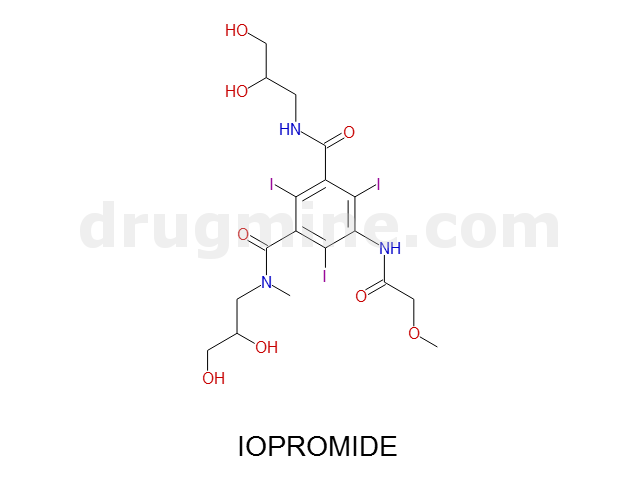

Name: IOPROMIDE
ID :
MW: 791
Number of atoms: 32
Molecular_Formula: C18H24I3N3O8
Alogp: -1.057
Indication class : Diagnostic Aid (radiopaque medium)
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
iopromide containing products summary
There are in total 8 different products containing the active ingredient iopromide. From the 8 drug products, zero have been discontinued.Product id = 11245
Application Number = 20220
Date of Application = 10,, May, 1995
RX/OTC/DISCN = RX
Tradename = ULTRAVIST 370
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 001
Tecode =
Rld = Yes
Strength = 76.9%
----
Product id = 11243
Application Number = 20220
Date of Application = 10,, May, 1995
RX/OTC/DISCN = RX
Tradename = ULTRAVIST 300
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 002
Tecode =
Rld = Yes
Strength = 62.3%
----
Product id = 11242
Application Number = 20220
Date of Application = 10,, May, 1995
RX/OTC/DISCN = RX
Tradename = ULTRAVIST 240
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 003
Tecode =
Rld = Yes
Strength = 49.9%
----
Product id = 11241
Application Number = 20220
Date of Application = 10,, May, 1995
RX/OTC/DISCN = RX
Tradename = ULTRAVIST 150
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 004
Tecode =
Rld = Yes
Strength = 31.2%
----
Product id = 11244
Application Number = 20220
Date of Application = 18,, Nov, 2008
RX/OTC/DISCN = RX
Tradename = ULTRAVIST 300 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 005
Tecode =
Rld = Yes
Strength = 62.3%
----
Product id = 11239
Application Number = 21425
Date of Application = 20,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = ULTRAVIST (PHARMACY BULK)
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 001
Tecode =
Rld = Yes
Strength = 62.3%
----
Product id = 11240
Application Number = 21425
Date of Application = 20,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = ULTRAVIST (PHARMACY BULK)
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 002
Tecode =
Rld = Yes
Strength = 76.9%
----
Product id = 11238
Application Number = 21425
Date of Application = 12,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = ULTRAVIST (PHARMACY BULK)
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 003
Tecode =
Rld = Yes
Strength = 49.9%
----