isoniazid
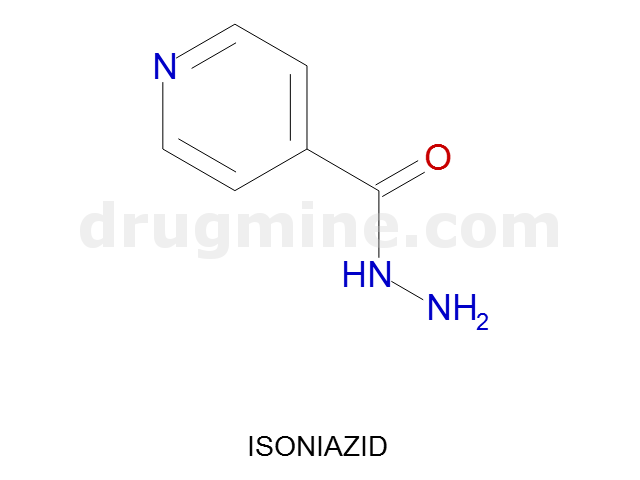
Name: ISONIAZID
ID :
MW: 137
Number of atoms: 10
Molecular_Formula: C6H7N3O
Alogp: -0.811
Indication class : Antibacterial (tuberculostatic)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
isoniazid containing products summary
There are in total 60 different products containing the active ingredient isoniazid. From the 60 drug products, 49 have been discontinued.Product id = 25520
Application Number = 8392
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NYDRAZID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 15144
Application Number = 8420
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RIMIFON
Route/format = ORAL / SYRUP
Application Type = N
Applicant Name = ROCHE
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/5ML
----
Product id = 10654
Application Number = 8420
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RIMIFON
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ROCHE
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 10655
Application Number = 8420
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RIMIFON
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ROCHE
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 23048
Application Number = 8428
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PANRAY
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 23049
Application Number = 8428
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PANRAY
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 23050
Application Number = 8428
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PANRAY
ProductNo = 003
Tecode =
Rld = No
Strength = 300MG
----
Product id = 23040
Application Number = 8499
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LILLY
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 23041
Application Number = 8499
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LILLY
ProductNo = 003
Tecode =
Rld = No
Strength = 300MG
----
Product id = 9755
Application Number = 8662
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NYDRAZID
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 23057
Application Number = 8678
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AA
Rld = Yes
Strength = 100MG
----
Product id = 23058
Application Number = 8678
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AA
Rld = Yes
Strength = 300MG
----
Product id = 23042
Application Number = 40090
Date of Application = 26,, Jun, 1997
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MIKART
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 23043
Application Number = 40090
Date of Application = 26,, Jun, 1997
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MIKART
ProductNo = 002
Tecode =
Rld = No
Strength = 300MG
----
Product id = 8520
Application Number = 40648
Date of Application = 5,, Jul, 2005
RX/OTC/DISCN = RX
Tradename = ISONIAZID
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = Yes
Strength = 100MG/ML
----
Product id = 27531
Application Number = 50705
Date of Application = 31,, May, 1994
RX/OTC/DISCN = RX
Tradename = RIFATER
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = Yes
Strength = 50MG;300MG;120MG
----
Product id = 3189
Application Number = 61884
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = RIFAMATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = Yes
Strength = 150MG;300MG
----
Product id = 3198
Application Number = 65221
Date of Application = 29,, Jul, 2005
RX/OTC/DISCN = DISCN
Tradename = RIFAMPIN AND ISONIAZID
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG;300MG
----
Product id = 23068
Application Number = 80120
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WHITEWORTH TOWN PLSN
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 28239
Application Number = 80126
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = STANOZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EVERYLIFE
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 28240
Application Number = 80126
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = STANOZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EVERYLIFE
ProductNo = 002
Tecode =
Rld = No
Strength = 300MG
----
Product id = 23055
Application Number = 80132
Date of Application = 14,, Jul, 1982
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG
----
Product id = 23056
Application Number = 80132
Date of Application = 14,, Jul, 1982
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 004
Tecode =
Rld = No
Strength = 100MG
----
Product id = 22687
Application Number = 80134
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYZYD
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MEDPOINTE PHARM HLC
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 22688
Application Number = 80134
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYZYD
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MEDPOINTE PHARM HLC
ProductNo = 004
Tecode =
Rld = No
Strength = 300MG
----
Product id = 23045
Application Number = 80136
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 23331
Application Number = 80140
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LANIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 23332
Application Number = 80140
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LANIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 23037
Application Number = 80153
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 23035
Application Number = 80212
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 23038
Application Number = 80270
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 20645
Application Number = 80330
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DOW-ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DOW PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 300MG
----
Product id = 23053
Application Number = 80368
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PHOENIX LABS NY
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 23054
Application Number = 80368
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PHOENIX LABS NY
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 23062
Application Number = 80401
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 23065
Application Number = 80521
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 23061
Application Number = 80522
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 23063
Application Number = 80523
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 22927
Application Number = 80935
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = INH
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 23030
Application Number = 80936
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AA
Rld = No
Strength = 100MG
----
Product id = 23031
Application Number = 80937
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 002
Tecode = AA
Rld = No
Strength = 300MG
----
Product id = 23044
Application Number = 80941
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MK LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 15015
Application Number = 81118
Date of Application = 21,, Jul, 1997
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = MIKART
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/5ML
----
Product id = 23051
Application Number = 83060
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 23066
Application Number = 83178
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 23039
Application Number = 83610
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 23034
Application Number = 83632
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 23046
Application Number = 83633
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 23047
Application Number = 84050
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NEXGEN PHARMA INC
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 23052
Application Number = 85091
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PHARMAVITE
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 23067
Application Number = 85784
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 23064
Application Number = 85790
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 23036
Application Number = 87425
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 23033
Application Number = 88119
Date of Application = 17,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 23032
Application Number = 88231
Date of Application = 17,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 15014
Application Number = 88235
Date of Application = 10,, Nov, 1983
RX/OTC/DISCN = RX
Tradename = ISONIAZID
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = CAROLINA MEDCL
ProductNo = 001
Tecode =
Rld = Yes
Strength = 50MG/5ML
----
Product id = 15017
Application Number = 89243
Date of Application = 3,, Feb, 1986
RX/OTC/DISCN = DISCN
Tradename = LANIAZID
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/5ML
----
Product id = 23333
Application Number = 89776
Date of Application = 13,, Jun, 1988
RX/OTC/DISCN = RX
Tradename = LANIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode = AA
Rld = No
Strength = 300MG
----
Product id = 23059
Application Number = 202610
Date of Application = 29,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = THEPHARMANETWORK LLC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 100MG
----
Product id = 23060
Application Number = 202610
Date of Application = 29,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = ISONIAZID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = THEPHARMANETWORK LLC
ProductNo = 002
Tecode = AA
Rld = No
Strength = 300MG
----