ketorolac-tromethamine
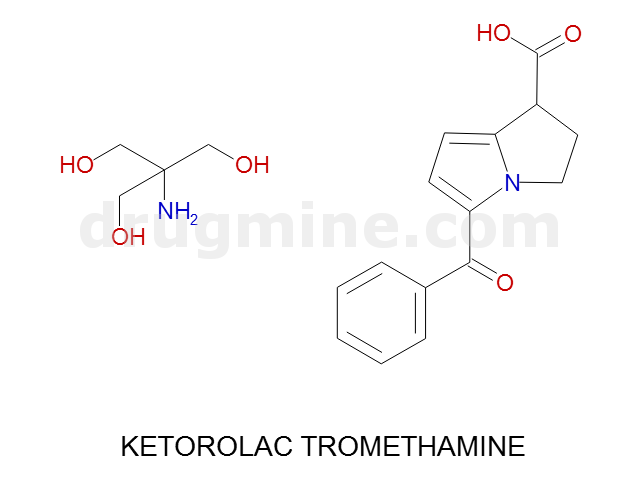
Name: KETOROLAC TROMETHAMINE
ID :
MW: 255
Number of atoms: 19
Molecular_Formula: C15H13NO3
Alogp: 2.832
Indication class : Analgesic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
ketorolac tromethamine containing products summary
There are in total 64 different products containing the active ingredient ketorolac tromethamine. From the 64 drug products, 34 have been discontinued.Product id = 28939
Application Number = 19645
Date of Application = 20,, Dec, 1991
RX/OTC/DISCN = DISCN
Tradename = TORADOL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROCHE PALO
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 11135
Application Number = 19698
Date of Application = 30,, Nov, 1989
RX/OTC/DISCN = DISCN
Tradename = TORADOL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ROCHE PALO
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG/ML
----
Product id = 11136
Application Number = 19698
Date of Application = 30,, Nov, 1989
RX/OTC/DISCN = DISCN
Tradename = TORADOL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ROCHE PALO
ProductNo = 002
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 12838
Application Number = 19700
Date of Application = 9,, Nov, 1992
RX/OTC/DISCN = RX
Tradename = ACULAR
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = ALLERGAN
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.5%
----
Product id = 12840
Application Number = 20811
Date of Application = 3,, Nov, 1997
RX/OTC/DISCN = DISCN
Tradename = ACULAR PRESERVATIVE FREE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = ALLERGAN
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 12839
Application Number = 21528
Date of Application = 30,, May, 2003
RX/OTC/DISCN = RX
Tradename = ACULAR LS
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = ALLERGAN
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.4%
----
Product id = 14395
Application Number = 22382
Date of Application = 14,, May, 2010
RX/OTC/DISCN = RX
Tradename = SPRIX
Route/format = NASAL / SPRAY, METERED
Application Type = N
Applicant Name = EGALET US INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 15.75MG/SPRAY
----
Product id = 12841
Application Number = 22427
Date of Application = 22,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = ACUVAIL
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = ALLERGAN
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.45%
----
Product id = 23179
Application Number = 74754
Date of Application = 16,, May, 1997
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23177
Application Number = 74761
Date of Application = 16,, May, 1997
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 10MG
----
Product id = 23176
Application Number = 74790
Date of Application = 26,, Jun, 1997
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CYCLE PHARMS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 8642
Application Number = 74801
Date of Application = 5,, Jun, 1997
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG/ML
----
Product id = 8645
Application Number = 74801
Date of Application = 5,, Jun, 1997
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 8643
Application Number = 74802
Date of Application = 5,, Jun, 1997
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 15MG/ML
----
Product id = 8646
Application Number = 74802
Date of Application = 5,, Jun, 1997
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = 30MG/ML
----
Product id = 23180
Application Number = 74955
Date of Application = 19,, Sep, 1997
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 8644
Application Number = 74993
Date of Application = 27,, Jan, 1999
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 15MG/ML
----
Product id = 8647
Application Number = 74993
Date of Application = 27,, Jan, 1999
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 30MG/ML
----
Product id = 8629
Application Number = 75222
Date of Application = 26,, Apr, 1999
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG/ML **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 8631
Application Number = 75222
Date of Application = 26,, Apr, 1999
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 002
Tecode =
Rld = No
Strength = 30MG/ML **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 8632
Application Number = 75228
Date of Application = 26,, Apr, 1999
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG/ML **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 8633
Application Number = 75230
Date of Application = 25,, Oct, 1999
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 8630
Application Number = 75230
Date of Application = 25,, Oct, 1999
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 002
Tecode =
Rld = No
Strength = 15MG/ML
----
Product id = 23178
Application Number = 75284
Date of Application = 23,, Jun, 1999
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 8638
Application Number = 75299
Date of Application = 3,, Nov, 1999
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG/ML
----
Product id = 8640
Application Number = 75299
Date of Application = 3,, Nov, 1999
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 002
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 8627
Application Number = 75348
Date of Application = 28,, Nov, 2000
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG/ML
----
Product id = 8628
Application Number = 75348
Date of Application = 28,, Nov, 2000
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 8624
Application Number = 75626
Date of Application = 24,, Jul, 2001
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 8625
Application Number = 75631
Date of Application = 29,, Jun, 2001
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 8623
Application Number = 75631
Date of Application = 29,, Jun, 2001
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode =
Rld = No
Strength = 15MG/ML
----
Product id = 8639
Application Number = 75772
Date of Application = 21,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG/ML
----
Product id = 8641
Application Number = 75772
Date of Application = 21,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 002
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 8634
Application Number = 75784
Date of Application = 11,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 15MG/ML
----
Product id = 8635
Application Number = 75784
Date of Application = 11,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 30MG/ML
----
Product id = 12999
Application Number = 76109
Date of Application = 5,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.5%
----
Product id = 8621
Application Number = 76209
Date of Application = 21,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AMPHASTAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG/ML
----
Product id = 8622
Application Number = 76209
Date of Application = 21,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AMPHASTAR PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 8652
Application Number = 76271
Date of Application = 6,, Oct, 2004
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG/ML
----
Product id = 8653
Application Number = 76271
Date of Application = 6,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AP
Rld = No
Strength = 30MG/ML
----
Product id = 12997
Application Number = 76583
Date of Application = 5,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = ALCON PHARMS LTD
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.5%
----
Product id = 8636
Application Number = 76722
Date of Application = 27,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAND PHARMA LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG/ML
----
Product id = 8637
Application Number = 76722
Date of Application = 27,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAND PHARMA LTD
ProductNo = 002
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 8626
Application Number = 77201
Date of Application = 14,, Oct, 2005
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 12998
Application Number = 77308
Date of Application = 5,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.4%
----
Product id = 8656
Application Number = 77942
Date of Application = 27,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AP
Rld = No
Strength = 15MG/ML
----
Product id = 8657
Application Number = 77942
Date of Application = 27,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 002
Tecode = AP
Rld = No
Strength = 30MG/ML
----
Product id = 8658
Application Number = 77943
Date of Application = 27,, Mar, 2007
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 8648
Application Number = 78145
Date of Application = 14,, Jan, 2008
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG/ML
----
Product id = 8649
Application Number = 78145
Date of Application = 14,, Jan, 2008
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 002
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 8617
Application Number = 78299
Date of Application = 16,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 001
Tecode = AP
Rld = No
Strength = 15MG/ML
----
Product id = 8619
Application Number = 78299
Date of Application = 16,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 002
Tecode = AP
Rld = No
Strength = 30MG/ML
----
Product id = 12993
Application Number = 78399
Date of Application = 5,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.4%
----
Product id = 12994
Application Number = 78434
Date of Application = 5,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.5%
----
Product id = 12996
Application Number = 78721
Date of Application = 5,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = ALCON PHARMS LTD
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.4%
----
Product id = 8654
Application Number = 78737
Date of Application = 6,, Oct, 2008
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG/ML
----
Product id = 8655
Application Number = 78737
Date of Application = 6,, Oct, 2008
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 002
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 13000
Application Number = 90017
Date of Application = 5,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.5%
----
Product id = 8650
Application Number = 91065
Date of Application = 27,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT PHARMS
ProductNo = 001
Tecode = AP
Rld = No
Strength = 15MG/ML
----
Product id = 8651
Application Number = 91065
Date of Application = 27,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT PHARMS
ProductNo = 002
Tecode = AP
Rld = No
Strength = 30MG/ML
----
Product id = 8618
Application Number = 201155
Date of Application = 4,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 001
Tecode = AP
Rld = No
Strength = 15MG/ML
----
Product id = 8620
Application Number = 201155
Date of Application = 4,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = KETOROLAC TROMETHAMINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 002
Tecode = AP
Rld = No
Strength = 30MG/ML
----
Product id = 12995
Application Number = 203376
Date of Application = 10,, Feb, 2014
RX/OTC/DISCN = DISCN
Tradename = KETOROLAC TROMETHAMINE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode =
Rld = No
Strength = 0.45%
----
Product id = 13645
Application Number = 205388
Date of Application = 30,, May, 2014
RX/OTC/DISCN = RX
Tradename = OMIDRIA
Route/format = IRRIGATION / SOLUTION
Application Type = N
Applicant Name = OMEROS
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 4.24MG BASE/ML;EQ 12.4MG BASE/ML
----