levalbuterol-hydrochloride
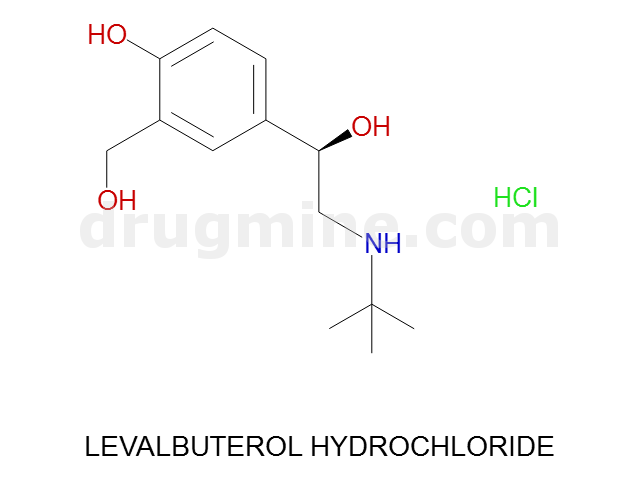
Name: LEVALBUTEROL HYDROCHLORIDE
ID :
MW: 239
Number of atoms: 17
Molecular_Formula: C13H21NO3
Alogp: 0.941
Indication class : Bronchodilator; Asthma Prophylactic
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
levalbuterol hydrochloride containing products summary
There are in total 18 different products containing the active ingredient levalbuterol hydrochloride. From the 18 drug products, zero have been discontinued.Product id = 13416
Application Number = 20837
Date of Application = 25,, Mar, 1999
RX/OTC/DISCN = RX
Tradename = XOPENEX
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = OAK PHARMS INC
ProductNo = 001
Tecode = AN
Rld = Yes
Strength = EQ 0.021% BASE
----
Product id = 13417
Application Number = 20837
Date of Application = 25,, Mar, 1999
RX/OTC/DISCN = RX
Tradename = XOPENEX
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = OAK PHARMS INC
ProductNo = 002
Tecode = AN
Rld = Yes
Strength = EQ 0.042% BASE
----
Product id = 13415
Application Number = 20837
Date of Application = 30,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = XOPENEX
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = OAK PHARMS INC
ProductNo = 003
Tecode = AN
Rld = Yes
Strength = EQ 0.0103% BASE
----
Product id = 13418
Application Number = 20837
Date of Application = 18,, Jul, 2003
RX/OTC/DISCN = RX
Tradename = XOPENEX
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = OAK PHARMS INC
ProductNo = 004
Tecode = AN
Rld = Yes
Strength = EQ 0.25% BASE
----
Product id = 13382
Application Number = 77756
Date of Application = 9,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = LEVALBUTEROL HYDROCHLORIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.021% BASE
----
Product id = 13383
Application Number = 77756
Date of Application = 9,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = LEVALBUTEROL HYDROCHLORIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 002
Tecode = AN
Rld = No
Strength = EQ 0.042% BASE
----
Product id = 13381
Application Number = 77756
Date of Application = 9,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = LEVALBUTEROL HYDROCHLORIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 003
Tecode = AN
Rld = No
Strength = EQ 0.0103% BASE
----
Product id = 13373
Application Number = 77800
Date of Application = 15,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = LEVALBUTEROL HYDROCHLORIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = MYLAN SPECLT
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.0103% BASE
----
Product id = 13374
Application Number = 77800
Date of Application = 15,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = LEVALBUTEROL HYDROCHLORIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = MYLAN SPECLT
ProductNo = 002
Tecode = AN
Rld = No
Strength = EQ 0.021% BASE
----
Product id = 13375
Application Number = 77800
Date of Application = 15,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = LEVALBUTEROL HYDROCHLORIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = MYLAN SPECLT
ProductNo = 003
Tecode = AN
Rld = No
Strength = EQ 0.042% BASE
----
Product id = 13372
Application Number = 78171
Date of Application = 13,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = LEVALBUTEROL HYDROCHLORIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.0103% BASE
----
Product id = 13370
Application Number = 78171
Date of Application = 13,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = LEVALBUTEROL HYDROCHLORIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 002
Tecode = AN
Rld = No
Strength = EQ 0.021% BASE
----
Product id = 13371
Application Number = 78171
Date of Application = 13,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = LEVALBUTEROL HYDROCHLORIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 003
Tecode = AN
Rld = No
Strength = EQ 0.042% BASE
----
Product id = 13376
Application Number = 78309
Date of Application = 20,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = LEVALBUTEROL HYDROCHLORIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = MYLAN SPECLT
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.25% BASE
----
Product id = 13378
Application Number = 90297
Date of Application = 26,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = LEVALBUTEROL HYDROCHLORIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.0103% BASE
----
Product id = 13379
Application Number = 90297
Date of Application = 26,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = LEVALBUTEROL HYDROCHLORIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AN
Rld = No
Strength = EQ 0.021% BASE
----
Product id = 13380
Application Number = 90297
Date of Application = 26,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = LEVALBUTEROL HYDROCHLORIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 003
Tecode = AN
Rld = No
Strength = EQ 0.042% BASE
----
Product id = 13377
Application Number = 200875
Date of Application = 11,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = LEVALBUTEROL HYDROCHLORIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = TEVA PARENTERAL
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.25% BASE
----