levocetirizine-dihydrochloride
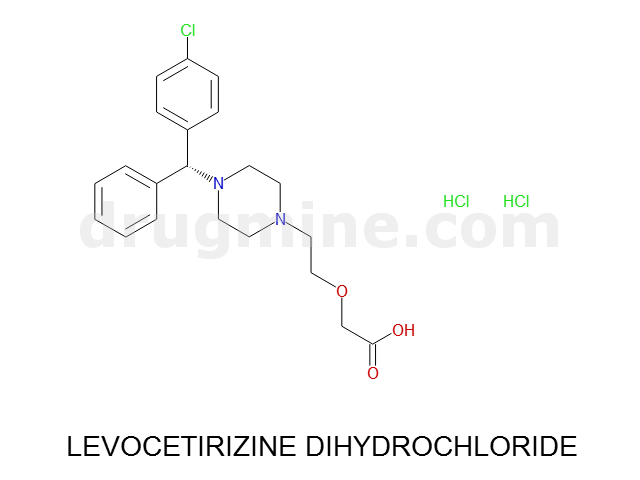

Name: LEVOCETIRIZINE DIHYDROCHLORIDE
ID :
MW: 389
Number of atoms: 27
Molecular_Formula: C21H25ClN2O3
Alogp: 0.453
Indication class : .
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
levocetirizine dihydrochloride containing products summary
There are in total 15 different products containing the active ingredient levocetirizine dihydrochloride. From the 15 drug products, 1 have been discontinued.Product id = 29749
Application Number = 22064
Date of Application = 25,, May, 2007
RX/OTC/DISCN = RX
Tradename = XYZAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = UCB INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 5MG
----
Product id = 14064
Application Number = 22157
Date of Application = 28,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = XYZAL
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = UCB INC
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 2.5MG/5ML
----
Product id = 23535
Application Number = 90199
Date of Application = 22,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = LEVOCETIRIZINE DIHYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23534
Application Number = 90229
Date of Application = 26,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = LEVOCETIRIZINE DIHYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SYNTHON PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23533
Application Number = 90362
Date of Application = 31,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = LEVOCETIRIZINE DIHYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23528
Application Number = 90385
Date of Application = 24,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = LEVOCETIRIZINE DIHYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23527
Application Number = 90392
Date of Application = 24,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = LEVOCETIRIZINE DIHYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23531
Application Number = 90486
Date of Application = 26,, Mar, 2013
RX/OTC/DISCN = DISCN
Tradename = LEVOCETIRIZINE DIHYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 13898
Application Number = 91263
Date of Application = 7,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = LEVOCETIRIZINE DIHYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = L PERRIGO CO
ProductNo = 001
Tecode = AA
Rld = No
Strength = 2.5MG/5ML
----
Product id = 23529
Application Number = 91264
Date of Application = 29,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = LEVOCETIRIZINE DIHYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HETERO LABS LTD III
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23530
Application Number = 202046
Date of Application = 17,, Sep, 2013
RX/OTC/DISCN = RX
Tradename = LEVOCETIRIZINE DIHYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MICRO LABS LTD INDIA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 13899
Application Number = 202673
Date of Application = 26,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = LEVOCETIRIZINE DIHYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = TARO PHARM INDS
ProductNo = 001
Tecode = AA
Rld = No
Strength = 2.5MG/5ML
----
Product id = 13897
Application Number = 202915
Date of Application = 21,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = LEVOCETIRIZINE DIHYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 2.5MG/5ML
----
Product id = 23526
Application Number = 203027
Date of Application = 13,, Feb, 2015
RX/OTC/DISCN = RX
Tradename = LEVOCETIRIZINE DIHYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23532
Application Number = 203646
Date of Application = 9,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = LEVOCETIRIZINE DIHYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCIEGEN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----