levodopa


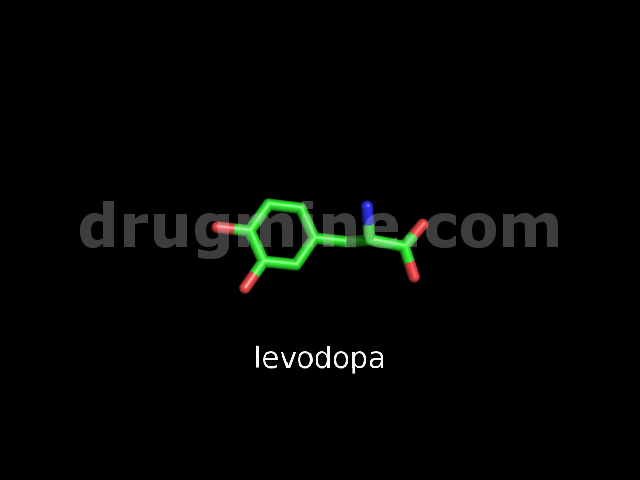
Name: LEVODOPA
ID :
MW: 197
Number of atoms: 14
Molecular_Formula: C9H11NO4
Alogp: -2.089
Indication class : Antiparkinsonian
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
levodopa containing products summary
There are in total 88 different products containing the active ingredient levodopa. From the 88 drug products, 33 have been discontinued.Product id = 2344
Application Number = 16912
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LARODOPA
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ROCHE
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 2343
Application Number = 16912
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LARODOPA
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ROCHE
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 23344
Application Number = 16912
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LARODOPA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROCHE
ProductNo = 003
Tecode =
Rld = No
Strength = 250MG
----
Product id = 23345
Application Number = 16912
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LARODOPA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROCHE
ProductNo = 004
Tecode =
Rld = No
Strength = 500MG
----
Product id = 23343
Application Number = 16912
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LARODOPA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROCHE
ProductNo = 005
Tecode =
Rld = No
Strength = 100MG
----
Product id = 2345
Application Number = 16912
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LARODOPA
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ROCHE
ProductNo = 006
Tecode =
Rld = No
Strength = 500MG
----
Product id = 1691
Application Number = 16913
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DOPAR
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = SHIRE
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 1692
Application Number = 16913
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DOPAR
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = SHIRE
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG
----
Product id = 1690
Application Number = 16913
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DOPAR
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = SHIRE
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 20637
Application Number = 16913
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DOPAR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SHIRE
ProductNo = 004
Tecode =
Rld = No
Strength = 250MG
----
Product id = 20638
Application Number = 16913
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DOPAR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SHIRE
ProductNo = 005
Tecode =
Rld = No
Strength = 500MG
----
Product id = 1132
Application Number = 16948
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BENDOPA
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = VALEANT PHARM INTL
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 1133
Application Number = 16948
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BENDOPA
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = VALEANT PHARM INTL
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG
----
Product id = 1131
Application Number = 16948
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BENDOPA
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = VALEANT PHARM INTL
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 28071
Application Number = 17555
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = SINEMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;100MG
----
Product id = 28073
Application Number = 17555
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = SINEMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 25MG;250MG
----
Product id = 28072
Application Number = 17555
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = SINEMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 16632
Application Number = 19856
Date of Application = 30,, May, 1991
RX/OTC/DISCN = RX
Tradename = SINEMET CR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 50MG;200MG
----
Product id = 16631
Application Number = 19856
Date of Application = 24,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = SINEMET CR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 28237
Application Number = 21485
Date of Application = 11,, Jun, 2003
RX/OTC/DISCN = RX
Tradename = STALEVO 50
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ORION PHARMA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 12.5MG;200MG;50MG
----
Product id = 28233
Application Number = 21485
Date of Application = 11,, Jun, 2003
RX/OTC/DISCN = RX
Tradename = STALEVO 100
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ORION PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;200MG;100MG
----
Product id = 28235
Application Number = 21485
Date of Application = 11,, Jun, 2003
RX/OTC/DISCN = RX
Tradename = STALEVO 150
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ORION PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 37.5MG;200MG;150MG
----
Product id = 28236
Application Number = 21485
Date of Application = 2,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = STALEVO 200
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ORION PHARMA
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = 50MG;200MG;200MG
----
Product id = 28238
Application Number = 21485
Date of Application = 29,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = STALEVO 75
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ORION PHARMA
ProductNo = 005
Tecode = AB
Rld = No
Strength = 18.75MG;200MG;75MG
----
Product id = 28234
Application Number = 21485
Date of Application = 29,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = STALEVO 125
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ORION PHARMA
ProductNo = 006
Tecode = AB
Rld = No
Strength = 31.25MG;200MG;125MG
----
Product id = 18986
Application Number = 73381
Date of Application = 28,, Sep, 1993
RX/OTC/DISCN = DISCN
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;100MG
----
Product id = 18987
Application Number = 73382
Date of Application = 28,, Sep, 1993
RX/OTC/DISCN = DISCN
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;100MG
----
Product id = 18988
Application Number = 73383
Date of Application = 28,, Sep, 1993
RX/OTC/DISCN = DISCN
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 18974
Application Number = 73586
Date of Application = 29,, Jun, 1995
RX/OTC/DISCN = DISCN
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;100MG
----
Product id = 18975
Application Number = 73587
Date of Application = 29,, Jun, 1995
RX/OTC/DISCN = DISCN
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;100MG
----
Product id = 18984
Application Number = 73589
Date of Application = 28,, Aug, 1992
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 18985
Application Number = 73607
Date of Application = 28,, Aug, 1992
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;250MG
----
Product id = 18983
Application Number = 73618
Date of Application = 28,, Aug, 1992
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;100MG
----
Product id = 18976
Application Number = 73620
Date of Application = 29,, Jun, 1995
RX/OTC/DISCN = DISCN
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 18977
Application Number = 74080
Date of Application = 25,, Mar, 1994
RX/OTC/DISCN = DISCN
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;100MG
----
Product id = 18978
Application Number = 74080
Date of Application = 25,, Mar, 1994
RX/OTC/DISCN = DISCN
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;100MG
----
Product id = 18979
Application Number = 74080
Date of Application = 25,, Mar, 1994
RX/OTC/DISCN = DISCN
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCS
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 18965
Application Number = 74260
Date of Application = 3,, Sep, 1993
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;100MG
----
Product id = 18966
Application Number = 74260
Date of Application = 3,, Sep, 1993
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 18967
Application Number = 74260
Date of Application = 3,, Sep, 1993
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;250MG
----
Product id = 15789
Application Number = 75091
Date of Application = 30,, Sep, 1999
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG;200MG
----
Product id = 15788
Application Number = 75091
Date of Application = 21,, Apr, 2000
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 15783
Application Number = 76212
Date of Application = 16,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 15784
Application Number = 76212
Date of Application = 16,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG;200MG
----
Product id = 15785
Application Number = 76521
Date of Application = 14,, May, 2004
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 15786
Application Number = 76521
Date of Application = 14,, May, 2004
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG;200MG
----
Product id = 16816
Application Number = 76643
Date of Application = 10,, Jun, 2005
RX/OTC/DISCN = DISCN
Tradename = CARBILEV
Route/format = ORAL / TABLET, FOR SUSPENSION
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;100MG
----
Product id = 16817
Application Number = 76643
Date of Application = 10,, Jun, 2005
RX/OTC/DISCN = DISCN
Tradename = CARBILEV
Route/format = ORAL / TABLET, FOR SUSPENSION
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;100MG
----
Product id = 16818
Application Number = 76643
Date of Application = 10,, Jun, 2005
RX/OTC/DISCN = DISCN
Tradename = CARBILEV
Route/format = ORAL / TABLET, FOR SUSPENSION
Application Type = A
Applicant Name = RANBAXY
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 15787
Application Number = 76663
Date of Application = 24,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = KV PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;200MG
----
Product id = 17021
Application Number = 76699
Date of Application = 27,, Aug, 2004
RX/OTC/DISCN = DISCN
Tradename = PARCOPA
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = UCB INC
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;100MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 17022
Application Number = 76699
Date of Application = 27,, Aug, 2004
RX/OTC/DISCN = DISCN
Tradename = PARCOPA
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = UCB INC
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;100MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 17023
Application Number = 76699
Date of Application = 27,, Aug, 2004
RX/OTC/DISCN = DISCN
Tradename = PARCOPA
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = UCB INC
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG;250MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 18968
Application Number = 77120
Date of Application = 2,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;100MG
----
Product id = 18969
Application Number = 77120
Date of Application = 2,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 18970
Application Number = 77120
Date of Application = 2,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;250MG
----
Product id = 15790
Application Number = 77828
Date of Application = 23,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARM INDS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 15791
Application Number = 77828
Date of Application = 23,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARM INDS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG;200MG
----
Product id = 18980
Application Number = 78536
Date of Application = 28,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;100MG
----
Product id = 18981
Application Number = 78536
Date of Application = 28,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 18982
Application Number = 78536
Date of Application = 28,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;250MG
----
Product id = 16858
Application Number = 78690
Date of Application = 31,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;100MG
----
Product id = 16859
Application Number = 78690
Date of Application = 31,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 16860
Application Number = 78690
Date of Application = 31,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;250MG
----
Product id = 16855
Application Number = 78893
Date of Application = 18,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;100MG
----
Product id = 16856
Application Number = 78893
Date of Application = 18,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 16857
Application Number = 78893
Date of Application = 18,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 25MG;250MG
----
Product id = 18989
Application Number = 79085
Date of Application = 10,, May, 2012
RX/OTC/DISCN = RX
Tradename = CARBIDOPA, LEVODOPA AND ENTACAPONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;200MG;100MG
----
Product id = 18990
Application Number = 79085
Date of Application = 10,, May, 2012
RX/OTC/DISCN = RX
Tradename = CARBIDOPA, LEVODOPA AND ENTACAPONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 002
Tecode = AB
Rld = No
Strength = 37.5MG;200MG;150MG
----
Product id = 18971
Application Number = 90324
Date of Application = 28,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;100MG
----
Product id = 18972
Application Number = 90324
Date of Application = 28,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 18973
Application Number = 90324
Date of Application = 28,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;250MG
----
Product id = 16852
Application Number = 90631
Date of Application = 8,, Jun, 2010
RX/OTC/DISCN = DISCN
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;100MG
----
Product id = 16853
Application Number = 90631
Date of Application = 8,, Jun, 2010
RX/OTC/DISCN = DISCN
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;100MG
----
Product id = 16854
Application Number = 90631
Date of Application = 8,, Jun, 2010
RX/OTC/DISCN = DISCN
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 18991
Application Number = 90786
Date of Application = 20,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = CARBIDOPA, LEVODOPA AND ENTACAPONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;200MG;50MG
----
Product id = 18992
Application Number = 90833
Date of Application = 20,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = CARBIDOPA, LEVODOPA AND ENTACAPONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 18.75MG;200MG;75MG
----
Product id = 18993
Application Number = 90833
Date of Application = 20,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = CARBIDOPA, LEVODOPA AND ENTACAPONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;200MG;100MG
----
Product id = 18994
Application Number = 90833
Date of Application = 20,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = CARBIDOPA, LEVODOPA AND ENTACAPONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 31.25MG;200MG;125MG
----
Product id = 18995
Application Number = 90833
Date of Application = 20,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = CARBIDOPA, LEVODOPA AND ENTACAPONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = 37.5MG;200MG;150MG
----
Product id = 18996
Application Number = 90833
Date of Application = 20,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = CARBIDOPA, LEVODOPA AND ENTACAPONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = 50MG;200MG;200MG
----
Product id = 15781
Application Number = 202323
Date of Application = 8,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 15782
Application Number = 202323
Date of Application = 8,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = CARBIDOPA AND LEVODOPA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG;200MG
----
Product id = 735
Application Number = 203312
Date of Application = 7,, Jan, 2015
RX/OTC/DISCN = RX
Tradename = RYTARY
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = IMPAX LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 23.75MG;95MG
----
Product id = 736
Application Number = 203312
Date of Application = 7,, Jan, 2015
RX/OTC/DISCN = RX
Tradename = RYTARY
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = IMPAX LABS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 36.25MG;145MG
----
Product id = 737
Application Number = 203312
Date of Application = 7,, Jan, 2015
RX/OTC/DISCN = RX
Tradename = RYTARY
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = IMPAX LABS INC
ProductNo = 003
Tecode =
Rld = No
Strength = 48.75MG;195MG
----
Product id = 738
Application Number = 203312
Date of Application = 7,, Jan, 2015
RX/OTC/DISCN = RX
Tradename = RYTARY
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = IMPAX LABS INC
ProductNo = 004
Tecode =
Rld = Yes
Strength = 61.25MG;245MG
----
Product id = 14608
Application Number = 203952
Date of Application = 9,, Jan, 2015
RX/OTC/DISCN = RX
Tradename = DUOPA
Route/format = ENTERAL / SUSPENSION
Application Type = N
Applicant Name = ABBVIE INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 4.63MG/ML;20MG/ML
----