lorazepam
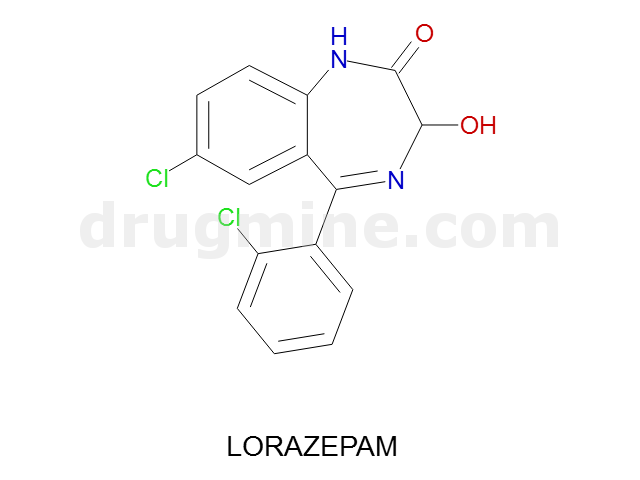

Name: LORAZEPAM
ID :
MW: 321
Number of atoms: 21
Molecular_Formula: C15H10Cl2N2O2
Alogp: 3.509
Indication class : Tranquilizer (minor)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
lorazepam containing products summary
There are in total 99 different products containing the active ingredient lorazepam. From the 99 drug products, 53 have been discontinued.Product id = 18177
Application Number = 17794
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = ATIVAN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = VALEANT INTL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 18178
Application Number = 17794
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = ATIVAN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = VALEANT INTL
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 18179
Application Number = 17794
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = ATIVAN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = VALEANT INTL
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 2MG
----
Product id = 6020
Application Number = 18140
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = ATIVAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 2MG/ML
----
Product id = 6021
Application Number = 18140
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = ATIVAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HIKMA MAPLE
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = 4MG/ML
----
Product id = 23876
Application Number = 70200
Date of Application = 9,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = LORAZ
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = QUANTUM PHARMICS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 23877
Application Number = 70201
Date of Application = 9,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = LORAZ
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = QUANTUM PHARMICS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 23878
Application Number = 70202
Date of Application = 9,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = LORAZ
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = QUANTUM PHARMICS
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 23894
Application Number = 70472
Date of Application = 10,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 23896
Application Number = 70473
Date of Application = 10,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 23898
Application Number = 70474
Date of Application = 10,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 23921
Application Number = 70539
Date of Application = 22,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 23922
Application Number = 70540
Date of Application = 22,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 23906
Application Number = 70675
Date of Application = 1,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 23907
Application Number = 70676
Date of Application = 1,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 23908
Application Number = 70677
Date of Application = 1,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 23879
Application Number = 70727
Date of Application = 7,, Mar, 1986
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AM THERAP
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 23880
Application Number = 70728
Date of Application = 7,, Mar, 1986
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AM THERAP
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 23881
Application Number = 70729
Date of Application = 7,, Mar, 1986
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AM THERAP
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 23926
Application Number = 71038
Date of Application = 12,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 23927
Application Number = 71039
Date of Application = 12,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 23928
Application Number = 71086
Date of Application = 23,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 23931
Application Number = 71087
Date of Application = 23,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 23934
Application Number = 71088
Date of Application = 23,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 23935
Application Number = 71110
Date of Application = 24,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 23929
Application Number = 71117
Date of Application = 24,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 23932
Application Number = 71118
Date of Application = 24,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 23916
Application Number = 71141
Date of Application = 21,, Apr, 1987
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 23912
Application Number = 71193
Date of Application = 15,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 23914
Application Number = 71194
Date of Application = 15,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 23917
Application Number = 71195
Date of Application = 15,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 23918
Application Number = 71245
Date of Application = 9,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 23919
Application Number = 71246
Date of Application = 9,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 23920
Application Number = 71247
Date of Application = 9,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 23913
Application Number = 71403
Date of Application = 21,, Apr, 1987
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 23915
Application Number = 71404
Date of Application = 21,, Apr, 1987
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 23888
Application Number = 71434
Date of Application = 1,, Sep, 1987
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 23889
Application Number = 71435
Date of Application = 1,, Sep, 1987
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 23890
Application Number = 71436
Date of Application = 1,, Sep, 1987
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 23900
Application Number = 71589
Date of Application = 13,, Oct, 1987
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 23902
Application Number = 71590
Date of Application = 13,, Oct, 1987
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 23904
Application Number = 71591
Date of Application = 13,, Oct, 1987
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 23895
Application Number = 72553
Date of Application = 29,, Mar, 1991
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 23897
Application Number = 72554
Date of Application = 29,, Mar, 1991
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 23899
Application Number = 72555
Date of Application = 29,, Mar, 1991
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 3945
Application Number = 72755
Date of Application = 28,, Jun, 1991
RX/OTC/DISCN = RX
Tradename = LORAZEPAM INTENSOL
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 2MG/ML
----
Product id = 23930
Application Number = 72926
Date of Application = 31,, Oct, 1991
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 23933
Application Number = 72927
Date of Application = 31,, Oct, 1991
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 23936
Application Number = 72928
Date of Application = 31,, Oct, 1991
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 8938
Application Number = 74243
Date of Application = 12,, Apr, 1994
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 2MG/ML
----
Product id = 8942
Application Number = 74243
Date of Application = 12,, Apr, 1994
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 4MG/ML
----
Product id = 8947
Application Number = 74276
Date of Application = 15,, Apr, 1994
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 8948
Application Number = 74276
Date of Application = 15,, Apr, 1994
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 4MG/ML
----
Product id = 8939
Application Number = 74280
Date of Application = 27,, May, 1994
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 8943
Application Number = 74280
Date of Application = 27,, May, 1994
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode =
Rld = No
Strength = 4MG/ML
----
Product id = 8940
Application Number = 74282
Date of Application = 27,, May, 1994
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 2MG/ML
----
Product id = 8944
Application Number = 74282
Date of Application = 27,, May, 1994
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 4MG/ML
----
Product id = 8941
Application Number = 74300
Date of Application = 12,, Apr, 1994
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 8945
Application Number = 74300
Date of Application = 19,, Mar, 1997
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 003
Tecode =
Rld = No
Strength = 4MG/ML
----
Product id = 8936
Application Number = 74496
Date of Application = 28,, Sep, 1998
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 8937
Application Number = 74496
Date of Application = 28,, Sep, 1998
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 002
Tecode =
Rld = No
Strength = 4MG/ML
----
Product id = 8950
Application Number = 74535
Date of Application = 12,, Sep, 1996
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 8952
Application Number = 74535
Date of Application = 12,, Sep, 1996
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 4MG/ML
----
Product id = 8951
Application Number = 74551
Date of Application = 12,, Sep, 1996
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 8953
Application Number = 74551
Date of Application = 12,, Sep, 1996
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 4MG/ML
----
Product id = 8949
Application Number = 74551
Date of Application = 12,, Sep, 1996
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 003
Tecode =
Rld = No
Strength = 1MG/0.5ML
----
Product id = 13918
Application Number = 74648
Date of Application = 18,, Mar, 1997
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG/5ML
----
Product id = 8934
Application Number = 74793
Date of Application = 16,, Mar, 2000
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 8935
Application Number = 74793
Date of Application = 16,, Mar, 2000
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 4MG/ML
----
Product id = 8930
Application Number = 74974
Date of Application = 23,, Jul, 1998
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 8931
Application Number = 75025
Date of Application = 23,, Jul, 1998
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode = AP
Rld = No
Strength = 2MG/ML
----
Product id = 23909
Application Number = 76045
Date of Application = 29,, Aug, 2001
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 23910
Application Number = 76045
Date of Application = 29,, Aug, 2001
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 23911
Application Number = 76045
Date of Application = 29,, Aug, 2001
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 8946
Application Number = 76150
Date of Application = 15,, Nov, 2004
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INTL MEDICATION SYS
ProductNo = 001
Tecode = AP
Rld = No
Strength = 2MG/ML
----
Product id = 8954
Application Number = 77074
Date of Application = 13,, Jul, 2005
RX/OTC/DISCN = RX
Tradename = LORAZEPAM PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 001
Tecode = AP
Rld = No
Strength = 2MG/ML
----
Product id = 8955
Application Number = 77074
Date of Application = 13,, Jul, 2005
RX/OTC/DISCN = RX
Tradename = LORAZEPAM PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 002
Tecode = AP
Rld = No
Strength = 4MG/ML
----
Product id = 8932
Application Number = 77076
Date of Application = 13,, Jul, 2005
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 8933
Application Number = 77076
Date of Application = 13,, Jul, 2005
RX/OTC/DISCN = DISCN
Tradename = LORAZEPAM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 002
Tecode =
Rld = No
Strength = 4MG/ML
----
Product id = 23891
Application Number = 77396
Date of Application = 13,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 23892
Application Number = 77396
Date of Application = 13,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 23893
Application Number = 77396
Date of Application = 13,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 23901
Application Number = 77657
Date of Application = 16,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 23903
Application Number = 77657
Date of Application = 16,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 23905
Application Number = 77657
Date of Application = 16,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 23923
Application Number = 77754
Date of Application = 10,, May, 2006
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 23924
Application Number = 77754
Date of Application = 10,, May, 2006
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 23925
Application Number = 77754
Date of Application = 10,, May, 2006
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 23885
Application Number = 78203
Date of Application = 30,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EXCELLIUM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 23886
Application Number = 78203
Date of Application = 30,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EXCELLIUM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 23887
Application Number = 78203
Date of Application = 30,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EXCELLIUM
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 23882
Application Number = 78826
Date of Application = 23,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 23883
Application Number = 78826
Date of Application = 23,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 23884
Application Number = 78826
Date of Application = 23,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 3943
Application Number = 79244
Date of Application = 28,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 2MG/ML
----
Product id = 3944
Application Number = 90260
Date of Application = 15,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = PHARM ASSOC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 2MG/ML
----
Product id = 3940
Application Number = 91383
Date of Application = 23,, Dec, 2009
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 001
Tecode = AA
Rld = No
Strength = 2MG/ML
----
Product id = 3942
Application Number = 91407
Date of Application = 19,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AA
Rld = No
Strength = 2MG/ML
----
Product id = 3941
Application Number = 200169
Date of Application = 30,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = LORAZEPAM
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = HI-TECH PHARMA CO
ProductNo = 001
Tecode = AA
Rld = No
Strength = 2MG/ML
----