mannitol
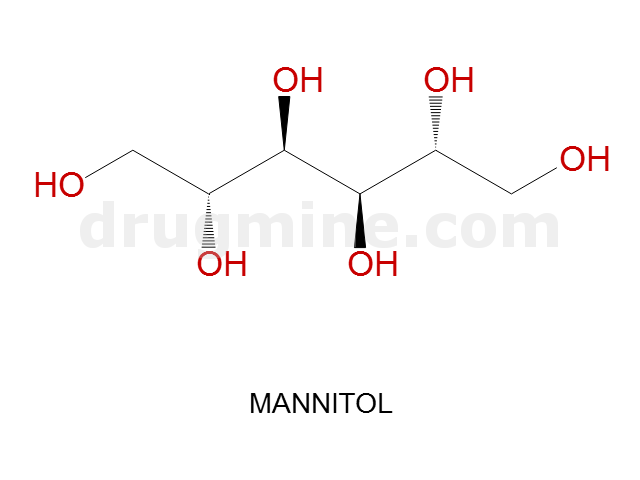


Name: MANNITOL
ID :
MW: 182
Number of atoms: 12
Molecular_Formula: C6H14O6
Alogp: -2.941
Indication class : Diagnostic Aid (renal function determination); Diuretic
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
mannitol containing products summary
There are in total 47 different products containing the active ingredient mannitol. From the 47 drug products, 18 have been discontinued.Product id = 9034
Application Number = 5620
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MANNITOL 25%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5GM/50ML
----
Product id = 9910
Application Number = 13684
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = OSMITROL 5% IN WATER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode = AP
Rld = No
Strength = 5GM/100ML
----
Product id = 9904
Application Number = 13684
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = OSMITROL 10% IN WATER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode = AP
Rld = No
Strength = 10GM/100ML
----
Product id = 9908
Application Number = 13684
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = OSMITROL 20% IN WATER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 003
Tecode = AP
Rld = No
Strength = 20GM/100ML
----
Product id = 9906
Application Number = 13684
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = OSMITROL 15% IN WATER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 004
Tecode = AP
Rld = No
Strength = 15GM/100ML
----
Product id = 9911
Application Number = 13684
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = OSMITROL 5% IN WATER IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 005
Tecode = AP
Rld = No
Strength = 5GM/100ML
----
Product id = 9905
Application Number = 13684
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = OSMITROL 10% IN WATER IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 006
Tecode = AP
Rld = No
Strength = 10GM/100ML
----
Product id = 9909
Application Number = 13684
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = OSMITROL 20% IN WATER IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 007
Tecode = AP
Rld = No
Strength = 20GM/100ML
----
Product id = 9907
Application Number = 13684
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = OSMITROL 15% IN WATER IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 008
Tecode = AP
Rld = No
Strength = 15GM/100ML
----
Product id = 9020
Application Number = 14738
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MANNITOL 20%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode =
Rld = No
Strength = 20GM/100ML
----
Product id = 9036
Application Number = 16080
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = MANNITOL 5%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode = AP
Rld = No
Strength = 5GM/100ML
----
Product id = 9008
Application Number = 16080
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = MANNITOL 10%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 002
Tecode = AP
Rld = No
Strength = 10GM/100ML
----
Product id = 9014
Application Number = 16080
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = MANNITOL 15%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 003
Tecode = AP
Rld = No
Strength = 15GM/100ML
----
Product id = 9021
Application Number = 16080
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = MANNITOL 20%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 004
Tecode = AP
Rld = No
Strength = 20GM/100ML
----
Product id = 9019
Application Number = 16080
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = MANNITOL 15% W/ DEXTROSE 5% IN SODIUM CHLORIDE 0.45%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 005
Tecode = AP
Rld = No
Strength = 15GM/100ML
----
Product id = 9013
Application Number = 16080
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = MANNITOL 10% W/ DEXTROSE 5% IN DISTILLED WATER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 006
Tecode = AP
Rld = No
Strength = 10GM/100ML
----
Product id = 9040
Application Number = 16080
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = MANNITOL 5% W/ DEXTROSE 5% IN SODIUM CHLORIDE 0.12%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 007
Tecode = AP
Rld = No
Strength = 5GM/100ML
----
Product id = 9037
Application Number = 16269
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MANNITOL 5%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 5GM/100ML
----
Product id = 9009
Application Number = 16269
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MANNITOL 10%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 002
Tecode =
Rld = No
Strength = 10GM/100ML
----
Product id = 9015
Application Number = 16269
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MANNITOL 15%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 003
Tecode =
Rld = No
Strength = 15GM/100ML
----
Product id = 9022
Application Number = 16269
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MANNITOL 20%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 004
Tecode =
Rld = No
Strength = 20GM/100ML
----
Product id = 9030
Application Number = 16269
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MANNITOL 25%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 005
Tecode =
Rld = No
Strength = 12.5GM/50ML
----
Product id = 9031
Application Number = 16269
Date of Application = 25,, Aug, 1994
RX/OTC/DISCN = RX
Tradename = MANNITOL 25%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 006
Tecode = AP
Rld = No
Strength = 12.5GM/50ML
----
Product id = 9010
Application Number = 16472
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MANNITOL 10%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MILES
ProductNo = 002
Tecode =
Rld = No
Strength = 10GM/100ML
----
Product id = 9023
Application Number = 16472
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MANNITOL 20%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MILES
ProductNo = 004
Tecode =
Rld = No
Strength = 20GM/100ML
----
Product id = 9016
Application Number = 16472
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MANNITOL 15%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MILES
ProductNo = 005
Tecode =
Rld = No
Strength = 15GM/100ML
----
Product id = 13651
Application Number = 16704
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESECTISOL
Route/format = IRRIGATION / SOLUTION
Application Type = N
Applicant Name = B BRAUN
ProductNo = 002
Tecode =
Rld = No
Strength = 5GM/100ML
----
Product id = 13652
Application Number = 16772
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = RESECTISOL IN PLASTIC CONTAINER
Route/format = IRRIGATION / SOLUTION
Application Type = N
Applicant Name = B BRAUN
ProductNo = 002
Tecode =
Rld = No
Strength = 5GM/100ML
----
Product id = 13671
Application Number = 17636
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SORBITOL-MANNITOL IN PLASTIC CONTAINER
Route/format = IRRIGATION / SOLUTION
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 540MG/100ML;2.7GM/100ML
----
Product id = 13672
Application Number = 18316
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = SORBITOL-MANNITOL IN PLASTIC CONTAINER
Route/format = IRRIGATION / SOLUTION
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 540MG/100ML;2.7GM/100ML
----
Product id = 9039
Application Number = 19603
Date of Application = 8,, Jan, 1987
RX/OTC/DISCN = RX
Tradename = MANNITOL 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 5GM/100ML
----
Product id = 9012
Application Number = 19603
Date of Application = 8,, Jan, 1987
RX/OTC/DISCN = RX
Tradename = MANNITOL 10% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 10GM/100ML
----
Product id = 9018
Application Number = 19603
Date of Application = 8,, Jan, 1990
RX/OTC/DISCN = RX
Tradename = MANNITOL 15% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 003
Tecode = AP
Rld = No
Strength = 15GM/100ML
----
Product id = 9025
Application Number = 19603
Date of Application = 8,, Jan, 1990
RX/OTC/DISCN = RX
Tradename = MANNITOL 20% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 004
Tecode = AP
Rld = No
Strength = 20GM/100ML
----
Product id = 9038
Application Number = 20006
Date of Application = 26,, Jul, 1993
RX/OTC/DISCN = RX
Tradename = MANNITOL 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode = AP
Rld = No
Strength = 5GM/100ML
----
Product id = 9011
Application Number = 20006
Date of Application = 26,, Jul, 1993
RX/OTC/DISCN = RX
Tradename = MANNITOL 10% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 002
Tecode = AP
Rld = No
Strength = 10GM/100ML
----
Product id = 9017
Application Number = 20006
Date of Application = 26,, Jul, 1993
RX/OTC/DISCN = RX
Tradename = MANNITOL 15% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 003
Tecode = AP
Rld = No
Strength = 15GM/100ML
----
Product id = 9024
Application Number = 20006
Date of Application = 26,, Jul, 1993
RX/OTC/DISCN = RX
Tradename = MANNITOL 20% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 004
Tecode = AP
Rld = No
Strength = 20GM/100ML
----
Product id = 12671
Application Number = 22368
Date of Application = 5,, Oct, 2010
RX/OTC/DISCN = DISCN
Tradename = ARIDOL KIT
Route/format = INHALATION / POWDER
Application Type = N
Applicant Name = PHARMAXIS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = N/A,5MG,10MG,20MG,40MG
----
Product id = 13670
Application Number = 80224
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SORBITOL-MANNITOL
Route/format = IRRIGATION / SOLUTION
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 540MG/100ML;2.7GM/100ML
----
Product id = 9029
Application Number = 80677
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = MANNITOL 25%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 12.5GM/50ML
----
Product id = 9032
Application Number = 83051
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = MANNITOL 25%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INTL MEDICATION
ProductNo = 001
Tecode = AP
Rld = No
Strength = 12.5GM/50ML
----
Product id = 9026
Application Number = 86754
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MANNITOL 25%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5GM/50ML
----
Product id = 9033
Application Number = 87409
Date of Application = 21,, Jan, 1982
RX/OTC/DISCN = RX
Tradename = MANNITOL 25%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 12.5GM/50ML
----
Product id = 9035
Application Number = 87460
Date of Application = 27,, Jun, 1983
RX/OTC/DISCN = DISCN
Tradename = MANNITOL 25%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5GM/50ML
----
Product id = 9027
Application Number = 89239
Date of Application = 6,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = MANNITOL 25%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5GM/50ML
----
Product id = 9028
Application Number = 89240
Date of Application = 6,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = MANNITOL 25%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5GM/50ML
----