metaproterenol-sulfate
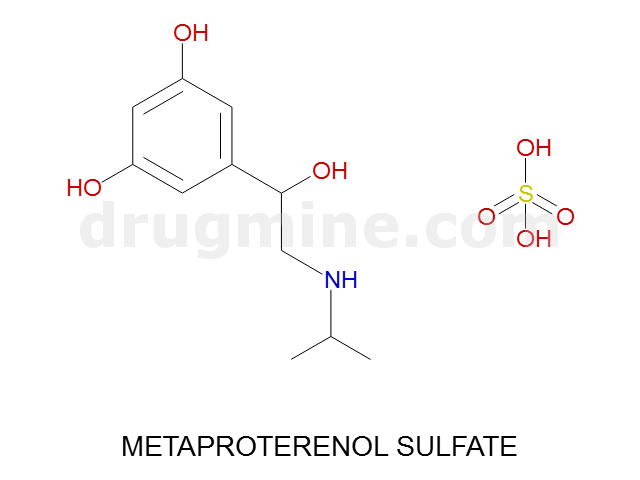

Name: METAPROTERENOL SULFATE
ID :
MW: 211
Number of atoms: 15
Molecular_Formula: C11H17NO3
Alogp: 1.099
Indication class : Bronchodilator
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
metaproterenol sulfate containing products summary
There are in total 39 different products containing the active ingredient metaproterenol sulfate. From the 39 drug products, 32 have been discontinued.Product id = 17590
Application Number = 15874
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ALUPENT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 17589
Application Number = 15874
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ALUPENT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 28
Application Number = 16402
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ALUPENT
Route/format = INHALATION / AEROSOL, METERED
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.65MG/INH
----
Product id = 14886
Application Number = 17571
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ALUPENT
Route/format = ORAL / SYRUP
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/5ML
----
Product id = 13293
Application Number = 17659
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ALUPENT
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 001
Tecode =
Rld = No
Strength = 5%
----
Product id = 13292
Application Number = 18761
Date of Application = 30,, Jun, 1983
RX/OTC/DISCN = DISCN
Tradename = ALUPENT
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.6%
----
Product id = 13291
Application Number = 18761
Date of Application = 10,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = ALUPENT
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 002
Tecode =
Rld = No
Strength = 0.4%
----
Product id = 13392
Application Number = 70804
Date of Application = 17,, Aug, 1987
RX/OTC/DISCN = RX
Tradename = METAPROTERENOL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = MYLAN SPECLT
ProductNo = 001
Tecode = AN
Rld = Yes
Strength = 0.6%
----
Product id = 13390
Application Number = 70805
Date of Application = 17,, Aug, 1987
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = DEY
ProductNo = 001
Tecode =
Rld = No
Strength = 5%
----
Product id = 24384
Application Number = 71013
Date of Application = 25,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 24385
Application Number = 71014
Date of Application = 25,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 13387
Application Number = 71018
Date of Application = 27,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.6%
----
Product id = 13386
Application Number = 71275
Date of Application = 27,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4%
----
Product id = 15032
Application Number = 71656
Date of Application = 13,, Oct, 1987
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = MORTON GROVE
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/5ML
----
Product id = 13394
Application Number = 71726
Date of Application = 14,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = NEPHRON
ProductNo = 001
Tecode =
Rld = No
Strength = 0.6%
----
Product id = 13391
Application Number = 71786
Date of Application = 5,, Aug, 1988
RX/OTC/DISCN = RX
Tradename = METAPROTERENOL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = MYLAN SPECLT
ProductNo = 001
Tecode = AN
Rld = Yes
Strength = 0.4%
----
Product id = 13389
Application Number = 71805
Date of Application = 5,, Aug, 1988
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = DEY
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 13388
Application Number = 71806
Date of Application = 5,, Aug, 1988
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = DEY
ProductNo = 001
Tecode =
Rld = No
Strength = 0.33%
----
Product id = 13393
Application Number = 71855
Date of Application = 14,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = NEPHRON
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4%
----
Product id = 15084
Application Number = 72023
Date of Application = 15,, Sep, 1988
RX/OTC/DISCN = DISCN
Tradename = PROMETA
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = MURO
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/5ML
----
Product id = 24380
Application Number = 72024
Date of Application = 28,, Jun, 1988
RX/OTC/DISCN = RX
Tradename = METAPROTERENOL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 24381
Application Number = 72025
Date of Application = 28,, Jun, 1988
RX/OTC/DISCN = RX
Tradename = METAPROTERENOL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = Yes
Strength = 20MG
----
Product id = 24378
Application Number = 72054
Date of Application = 23,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AM THERAP
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 24379
Application Number = 72055
Date of Application = 23,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AM THERAP
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 13397
Application Number = 72190
Date of Application = 7,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode =
Rld = No
Strength = 5%
----
Product id = 24382
Application Number = 72519
Date of Application = 30,, Mar, 1990
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 24383
Application Number = 72520
Date of Application = 30,, Mar, 1990
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 15034
Application Number = 72761
Date of Application = 27,, Feb, 1992
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/5ML
----
Product id = 24387
Application Number = 72795
Date of Application = 31,, Jan, 1991
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 24386
Application Number = 73013
Date of Application = 31,, Jan, 1991
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 15035
Application Number = 73034
Date of Application = 30,, Aug, 1991
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/5ML
----
Product id = 13400
Application Number = 73340
Date of Application = 30,, Mar, 1992
RX/OTC/DISCN = DISCN
Tradename = PROMETA
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = MURO
ProductNo = 001
Tecode =
Rld = No
Strength = 5%
----
Product id = 15033
Application Number = 73632
Date of Application = 22,, Jul, 1992
RX/OTC/DISCN = RX
Tradename = METAPROTERENOL SULFATE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = SILARX
ProductNo = 001
Tecode =
Rld = Yes
Strength = 10MG/5ML
----
Product id = 15036
Application Number = 74702
Date of Application = 24,, Mar, 1997
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/5ML
----
Product id = 15031
Application Number = 75235
Date of Application = 27,, Jan, 2000
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/5ML
----
Product id = 13384
Application Number = 75402
Date of Application = 28,, Feb, 2001
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4%
----
Product id = 13385
Application Number = 75403
Date of Application = 28,, Feb, 2001
RX/OTC/DISCN = DISCN
Tradename = METAPROTERENOL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 0.6%
----
Product id = 13395
Application Number = 75586
Date of Application = 30,, May, 2002
RX/OTC/DISCN = RX
Tradename = METAPROTERENOL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AN
Rld = No
Strength = 0.4%
----
Product id = 13396
Application Number = 75586
Date of Application = 30,, May, 2002
RX/OTC/DISCN = RX
Tradename = METAPROTERENOL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 002
Tecode = AN
Rld = No
Strength = 0.6%
----