metolazone
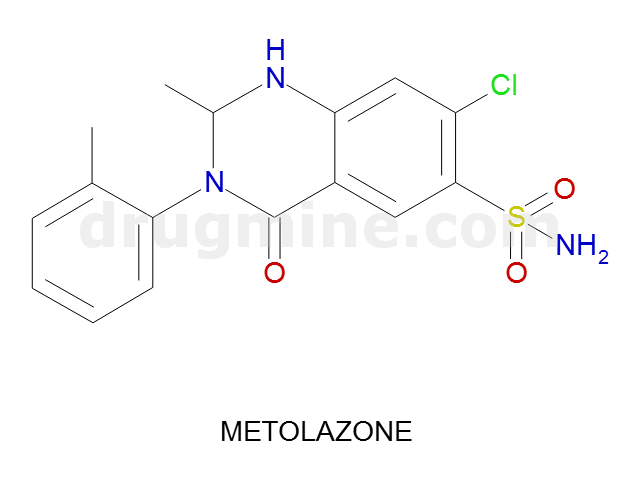

Name: METOLAZONE
ID :
MW: 366
Number of atoms: 24
Molecular_Formula: C16H16ClN3O3S
Alogp: 2.463
Indication class : Antihypertensive; Diuretic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
metolazone containing products summary
There are in total 18 different products containing the active ingredient metolazone. From the 18 drug products, 9 have been discontinued.Product id = 29762
Application Number = 17386
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = ZAROXOLYN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = UCB INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 29763
Application Number = 17386
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = ZAROXOLYN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = UCB INC
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 5MG
----
Product id = 29764
Application Number = 17386
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = ZAROXOLYN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = UCB INC
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 10MG
----
Product id = 20570
Application Number = 18535
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DIULO
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GD SEARLE LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 20571
Application Number = 18535
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DIULO
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GD SEARLE LLC
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG
----
Product id = 20572
Application Number = 18535
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DIULO
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GD SEARLE LLC
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG
----
Product id = 25124
Application Number = 19532
Date of Application = 30,, Oct, 1987
RX/OTC/DISCN = DISCN
Tradename = MYKROX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = UCB INC
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 24817
Application Number = 75543
Date of Application = 24,, Dec, 2003
RX/OTC/DISCN = DISCN
Tradename = METOLAZONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG
----
Product id = 24813
Application Number = 76466
Date of Application = 19,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = METOLAZONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 24814
Application Number = 76466
Date of Application = 19,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = METOLAZONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24811
Application Number = 76482
Date of Application = 29,, Apr, 2004
RX/OTC/DISCN = DISCN
Tradename = METOLAZONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 24815
Application Number = 76600
Date of Application = 6,, Jan, 2004
RX/OTC/DISCN = DISCN
Tradename = METOLAZONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 24808
Application Number = 76698
Date of Application = 23,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = METOLAZONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 24809
Application Number = 76698
Date of Application = 19,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = METOLAZONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 24810
Application Number = 76698
Date of Application = 19,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = METOLAZONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24812
Application Number = 76732
Date of Application = 19,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = METOLAZONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 24816
Application Number = 76833
Date of Application = 1,, Mar, 2004
RX/OTC/DISCN = DISCN
Tradename = METOLAZONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 24818
Application Number = 76891
Date of Application = 21,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = METOLAZONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----