metoprolol-tartrate
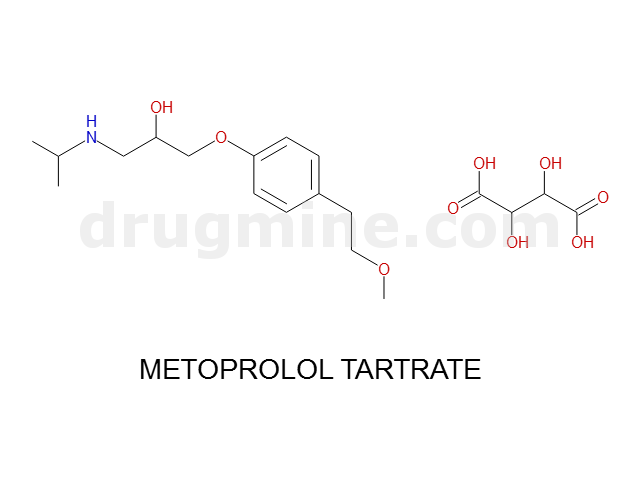
Name: METOPROLOL TARTRATE
ID :
MW: 267
Number of atoms: 19
Molecular_Formula: C15H25NO3
Alogp: 1.757
Indication class : Anti-Adrenergic (beta-receptor)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
metoprolol tartrate containing products summary
There are in total 69 different products containing the active ingredient metoprolol tartrate. From the 69 drug products, 16 have been discontinued.Product id = 23859
Application Number = 17963
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = LOPRESSOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 23860
Application Number = 17963
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = LOPRESSOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 23861
Application Number = 18303
Date of Application = 31,, Dec, 1984
RX/OTC/DISCN = RX
Tradename = LOPRESSOR HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;50MG
----
Product id = 23862
Application Number = 18303
Date of Application = 31,, Dec, 1984
RX/OTC/DISCN = RX
Tradename = LOPRESSOR HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 25MG;100MG
----
Product id = 23863
Application Number = 18303
Date of Application = 31,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = LOPRESSOR HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG;100MG
----
Product id = 8929
Application Number = 18704
Date of Application = 30,, Mar, 1984
RX/OTC/DISCN = RX
Tradename = LOPRESSOR
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 1MG/ML
----
Product id = 2398
Application Number = 19451
Date of Application = 31,, Dec, 1987
RX/OTC/DISCN = DISCN
Tradename = LOPRESSIDONE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;100MG
----
Product id = 2399
Application Number = 19451
Date of Application = 31,, Dec, 1987
RX/OTC/DISCN = DISCN
Tradename = LOPRESSIDONE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;200MG
----
Product id = 24846
Application Number = 73288
Date of Application = 25,, Mar, 1994
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 24847
Application Number = 73289
Date of Application = 25,, Mar, 1994
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 24832
Application Number = 73653
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 24833
Application Number = 73654
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 24831
Application Number = 73654
Date of Application = 15,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 24835
Application Number = 73666
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 24837
Application Number = 73666
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 9294
Application Number = 74032
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = METOPROLOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 9287
Application Number = 74133
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 24851
Application Number = 74141
Date of Application = 31,, Jan, 1995
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 24853
Application Number = 74141
Date of Application = 31,, Jan, 1995
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 24852
Application Number = 74143
Date of Application = 30,, Sep, 1994
RX/OTC/DISCN = DISCN
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 24854
Application Number = 74143
Date of Application = 30,, Sep, 1994
RX/OTC/DISCN = DISCN
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 24857
Application Number = 74217
Date of Application = 27,, May, 1994
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 24858
Application Number = 74217
Date of Application = 27,, May, 1994
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 24823
Application Number = 74258
Date of Application = 27,, Jan, 1994
RX/OTC/DISCN = DISCN
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 24824
Application Number = 74258
Date of Application = 27,, Jan, 1994
RX/OTC/DISCN = DISCN
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 24855
Application Number = 74333
Date of Application = 27,, Jan, 1994
RX/OTC/DISCN = DISCN
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 24856
Application Number = 74333
Date of Application = 27,, Jan, 1994
RX/OTC/DISCN = DISCN
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 24841
Application Number = 74380
Date of Application = 29,, Jul, 1994
RX/OTC/DISCN = DISCN
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 24842
Application Number = 74380
Date of Application = 29,, Jul, 1994
RX/OTC/DISCN = DISCN
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 24839
Application Number = 74453
Date of Application = 27,, Apr, 1995
RX/OTC/DISCN = DISCN
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 24840
Application Number = 74453
Date of Application = 27,, Apr, 1995
RX/OTC/DISCN = DISCN
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 24849
Application Number = 74644
Date of Application = 10,, Dec, 1996
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 24850
Application Number = 74644
Date of Application = 10,, Dec, 1996
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 9288
Application Number = 75160
Date of Application = 6,, Jul, 1998
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 9282
Application Number = 76495
Date of Application = 7,, Jul, 2003
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 24848
Application Number = 76670
Date of Application = 15,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 24834
Application Number = 76704
Date of Application = 16,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 24836
Application Number = 76704
Date of Application = 16,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 24838
Application Number = 76704
Date of Application = 16,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 100MG
----
Product id = 24862
Application Number = 76792
Date of Application = 20,, Aug, 2004
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;50MG
----
Product id = 24863
Application Number = 76792
Date of Application = 20,, Aug, 2004
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 24864
Application Number = 76792
Date of Application = 20,, Aug, 2004
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG;100MG
----
Product id = 9293
Application Number = 77360
Date of Application = 2,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 24825
Application Number = 77739
Date of Application = 11,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 24826
Application Number = 77739
Date of Application = 11,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 24827
Application Number = 77739
Date of Application = 11,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 9286
Application Number = 77761
Date of Application = 30,, May, 2007
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 9289
Application Number = 78085
Date of Application = 29,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 24828
Application Number = 78459
Date of Application = 17,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 24829
Application Number = 78459
Date of Application = 17,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 24830
Application Number = 78459
Date of Application = 17,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 9283
Application Number = 78950
Date of Application = 29,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CLARIS LIFESCIENCES
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 9292
Application Number = 90317
Date of Application = 19,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT STRIDES
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 9290
Application Number = 90386
Date of Application = 30,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 24865
Application Number = 90654
Date of Application = 19,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;50MG
----
Product id = 24866
Application Number = 90654
Date of Application = 19,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 24867
Application Number = 90654
Date of Application = 19,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG;100MG
----
Product id = 9284
Application Number = 91045
Date of Application = 25,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 9291
Application Number = 91307
Date of Application = 29,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 24843
Application Number = 200981
Date of Application = 28,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RUBICON RES PVT LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 24844
Application Number = 200981
Date of Application = 28,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RUBICON RES PVT LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 24845
Application Number = 200981
Date of Application = 28,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RUBICON RES PVT LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 24859
Application Number = 202870
Date of Application = 6,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;50MG
----
Product id = 24860
Application Number = 202870
Date of Application = 6,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 24861
Application Number = 202870
Date of Application = 6,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG;100MG
----
Product id = 24820
Application Number = 202871
Date of Application = 28,, May, 2013
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 24821
Application Number = 202871
Date of Application = 28,, May, 2013
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 24822
Application Number = 202871
Date of Application = 28,, May, 2013
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 9285
Application Number = 204205
Date of Application = 27,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAND PHARMA LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----