midazolam-hydrochloride
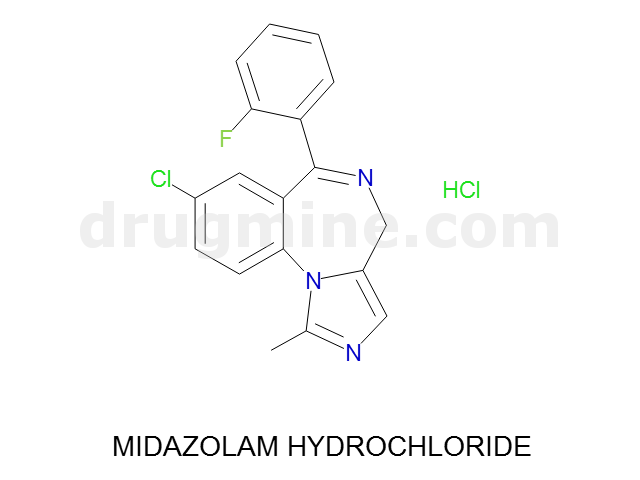
Name: MIDAZOLAM HYDROCHLORIDE
ID :
MW: 326
Number of atoms: 23
Molecular_Formula: C18H13ClFN3
Alogp: 4.126
Indication class : Anesthetic (injectable)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
midazolam hydrochloride containing products summary
There are in total 56 different products containing the active ingredient midazolam hydrochloride. From the 56 drug products, 21 have been discontinued.Product id = 11369
Application Number = 18654
Date of Application = 20,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = VERSED
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HLR
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 11368
Application Number = 18654
Date of Application = 26,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = VERSED
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HLR
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 15180
Application Number = 20942
Date of Application = 15,, Oct, 1998
RX/OTC/DISCN = DISCN
Tradename = VERSED
Route/format = ORAL / SYRUP
Application Type = N
Applicant Name = ROCHE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9348
Application Number = 75154
Date of Application = 20,, Jun, 2000
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9347
Application Number = 75154
Date of Application = 20,, Jun, 2000
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9351
Application Number = 75243
Date of Application = 20,, Jun, 2000
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9353
Application Number = 75243
Date of Application = 20,, Jun, 2000
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9342
Application Number = 75247
Date of Application = 23,, Jun, 2000
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9341
Application Number = 75247
Date of Application = 23,, Jun, 2000
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9343
Application Number = 75249
Date of Application = 23,, Jun, 2000
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9340
Application Number = 75263
Date of Application = 26,, Jun, 2000
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9355
Application Number = 75293
Date of Application = 20,, Jun, 2000
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 1MG BASE/ML
----
Product id = 9358
Application Number = 75293
Date of Application = 20,, Jun, 2000
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 5MG BASE/ML
----
Product id = 9352
Application Number = 75324
Date of Application = 20,, Jun, 2000
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9354
Application Number = 75324
Date of Application = 20,, Jun, 2000
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9356
Application Number = 75396
Date of Application = 20,, Jun, 2000
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9359
Application Number = 75396
Date of Application = 20,, Jun, 2000
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9363
Application Number = 75409
Date of Application = 20,, Jun, 2000
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9362
Application Number = 75409
Date of Application = 20,, Jun, 2000
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9345
Application Number = 75421
Date of Application = 20,, Jun, 2000
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9344
Application Number = 75421
Date of Application = 20,, Jun, 2000
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9346
Application Number = 75455
Date of Application = 20,, Jun, 2000
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEN VENUE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9333
Application Number = 75481
Date of Application = 30,, Jun, 2000
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AKORN INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9360
Application Number = 75484
Date of Application = 20,, Jun, 2000
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9332
Application Number = 75494
Date of Application = 30,, Jun, 2000
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AKORN INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9334
Application Number = 75494
Date of Application = 30,, Jun, 2000
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AKORN INC
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9337
Application Number = 75620
Date of Application = 1,, Nov, 2000
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9338
Application Number = 75620
Date of Application = 1,, Nov, 2000
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9335
Application Number = 75637
Date of Application = 31,, Oct, 2000
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9336
Application Number = 75637
Date of Application = 31,, Oct, 2000
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9339
Application Number = 75641
Date of Application = 19,, Oct, 2000
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9357
Application Number = 75856
Date of Application = 13,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9361
Application Number = 75856
Date of Application = 13,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9374
Application Number = 75857
Date of Application = 22,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 1MG BASE/ML
----
Product id = 9375
Application Number = 75857
Date of Application = 22,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 5MG BASE/ML
----
Product id = 15042
Application Number = 75873
Date of Application = 30,, Apr, 2002
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = EQ 2MG BASE/ML
----
Product id = 15039
Application Number = 75958
Date of Application = 4,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = HI TECH PHARMA
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9366
Application Number = 76020
Date of Application = 16,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INTL MEDICATION
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9367
Application Number = 76020
Date of Application = 16,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INTL MEDICATION
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 15041
Application Number = 76058
Date of Application = 15,, Mar, 2002
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9364
Application Number = 76144
Date of Application = 26,, Jan, 2005
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INTL MEDICATED
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9365
Application Number = 76144
Date of Application = 26,, Jan, 2005
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INTL MEDICATED
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 15040
Application Number = 76379
Date of Application = 2,, May, 2005
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 15038
Application Number = 77115
Date of Application = 9,, Sep, 2005
RX/OTC/DISCN = DISCN
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9368
Application Number = 78141
Date of Application = 30,, May, 2008
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9370
Application Number = 78141
Date of Application = 30,, May, 2008
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9369
Application Number = 78511
Date of Application = 10,, Nov, 2008
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9371
Application Number = 78511
Date of Application = 10,, Nov, 2008
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9376
Application Number = 90315
Date of Application = 29,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT STRIDES
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9377
Application Number = 90315
Date of Application = 29,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT STRIDES
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9378
Application Number = 90316
Date of Application = 4,, May, 2011
RX/OTC/DISCN = RX
Tradename = MIDOZALAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT STRIDES
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9379
Application Number = 90316
Date of Application = 4,, May, 2011
RX/OTC/DISCN = RX
Tradename = MIDOZALAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT STRIDES
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9349
Application Number = 90696
Date of Application = 29,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAND PHARMA LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9350
Application Number = 90850
Date of Application = 25,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAND PHARMA LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9372
Application Number = 203460
Date of Application = 22,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BD RX
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML
----
Product id = 9373
Application Number = 203460
Date of Application = 22,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = MIDAZOLAM HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BD RX
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----