mivacurium-chloride
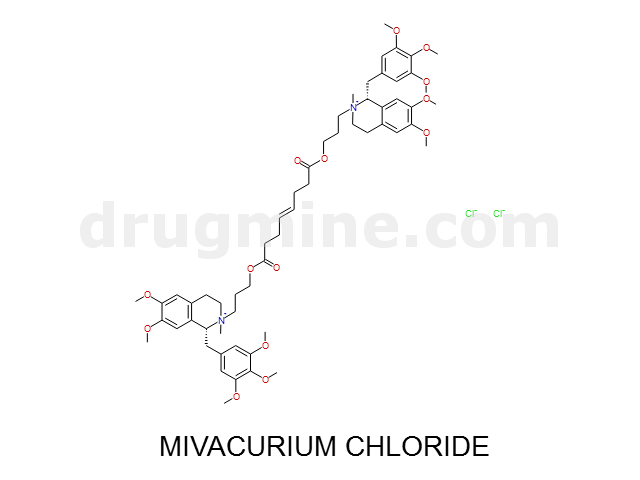

Name: MIVACURIUM CHLORIDE
ID :
MW: 1029
Number of atoms: 74
Molecular_Formula: C58H80N2O14
Alogp: 6.184
Indication class : Neuromuscular Blocking Agent
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
mivacurium chloride containing products summary
There are in total 6 different products containing the active ingredient mivacurium chloride. From the 6 drug products, 4 have been discontinued.Product id = 13568
Application Number = 20098
Date of Application = 22,, Jan, 1992
RX/OTC/DISCN = DISCN
Tradename = MIVACRON
Route/format = INTRAVENOUS / SOLUTION
Application Type = N
Applicant Name = ABBVIE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/ML (EQ 2MG BASE/ML)
----
Product id = 9436
Application Number = 20098
Date of Application = 22,, Jan, 1992
RX/OTC/DISCN = DISCN
Tradename = MIVACRON IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 0.5MG BASE/ML
----
Product id = 9437
Application Number = 20098
Date of Application = 22,, Jan, 1992
RX/OTC/DISCN = DISCN
Tradename = MIVACRON IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 50MG BASE/100ML
----
Product id = 13569
Application Number = 20098
Date of Application = 22,, Jan, 1992
RX/OTC/DISCN = RX
Tradename = MIVACRON
Route/format = INTRAVENOUS / SOLUTION
Application Type = N
Applicant Name = ABBVIE
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 10MG BASE/5ML (EQ 2MG BASE/ML)
----
Product id = 13570
Application Number = 20098
Date of Application = 22,, Jan, 1992
RX/OTC/DISCN = RX
Tradename = MIVACRON
Route/format = INTRAVENOUS / SOLUTION
Application Type = N
Applicant Name = ABBVIE
ProductNo = 005
Tecode =
Rld = Yes
Strength = EQ 20MG BASE/10ML (EQ 2MG BASE/ML)
----
Product id = 9438
Application Number = 78562
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = DISCN
Tradename = MIVACURIUM CHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/ML
----