moxifloxacin-hydrochloride
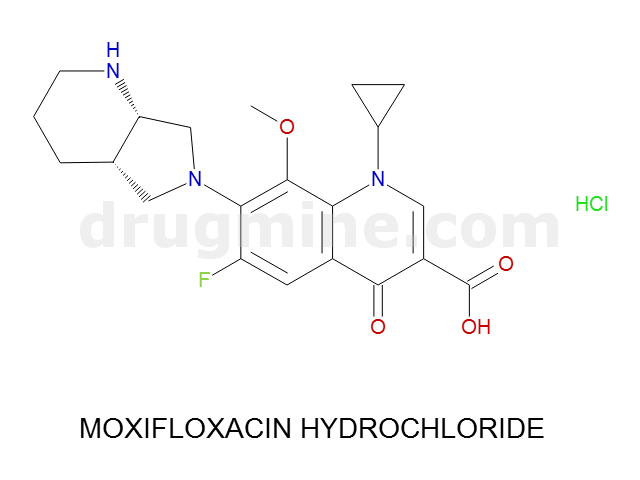
Name: MOXIFLOXACIN HYDROCHLORIDE
ID :
MW: 401
Number of atoms: 29
Molecular_Formula: C21H24FN3O4
Alogp: -0.702
Indication class : .
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
moxifloxacin hydrochloride containing products summary
There are in total 10 different products containing the active ingredient moxifloxacin hydrochloride. From the 10 drug products, zero have been discontinued.Product id = 18238
Application Number = 21085
Date of Application = 10,, Dec, 1999
RX/OTC/DISCN = RX
Tradename = AVELOX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 400MG BASE
----
Product id = 11745
Application Number = 21277
Date of Application = 30,, Nov, 2001
RX/OTC/DISCN = RX
Tradename = AVELOX IN SODIUM CHLORIDE 0.8% IN PLASTIC CONTAINER
Route/format = IV (INFUSION) / INJECTABLE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 001
Tecode =
Rld = Yes
Strength = 160MG/100ML
----
Product id = 13193
Application Number = 21598
Date of Application = 15,, Apr, 2003
RX/OTC/DISCN = RX
Tradename = VIGAMOX
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = ALCON PHARMS LTD
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = EQ 0.5% BASE
----
Product id = 13026
Application Number = 22428
Date of Application = 19,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = MOXEZA
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = ALCON PHARMS LTD
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 0.5% BASE
----
Product id = 25102
Application Number = 76938
Date of Application = 4,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = MOXIFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 400MG BASE
----
Product id = 25103
Application Number = 77437
Date of Application = 18,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = MOXIFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 400MG BASE
----
Product id = 25104
Application Number = 200160
Date of Application = 3,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = MOXIFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 400MG BASE
----
Product id = 13028
Application Number = 202525
Date of Application = 6,, Mar, 2015
RX/OTC/DISCN = RX
Tradename = MOXIFLOXACIN HYDROCHLORIDE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 25101
Application Number = 202632
Date of Application = 4,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = MOXIFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 400MG BASE
----
Product id = 13027
Application Number = 202867
Date of Application = 4,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = MOXIFLOXACIN HYDROCHLORIDE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.5% BASE
----