nadolol
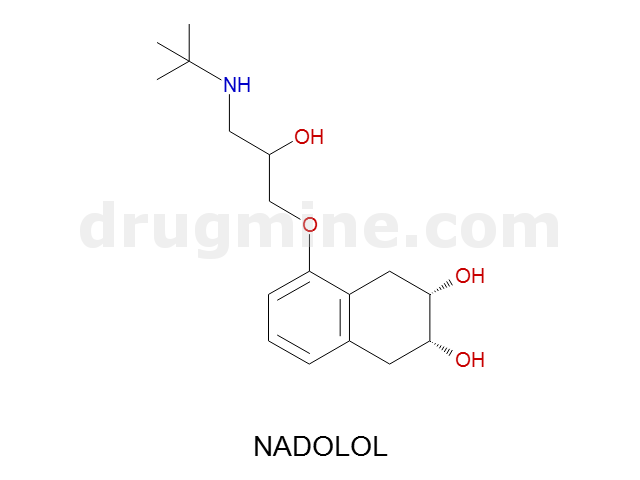
Name: NADOLOL
ID :
MW: 309
Number of atoms: 22
Molecular_Formula: C17H27NO4
Alogp: 1.146
Indication class : Anti-Adrenergic (beta-receptor)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
nadolol containing products summary
There are in total 25 different products containing the active ingredient nadolol. From the 25 drug products, 7 have been discontinued.Product id = 19985
Application Number = 18063
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CORGARD
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = KING PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 19986
Application Number = 18063
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CORGARD
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = KING PHARMS
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 80MG
----
Product id = 19987
Application Number = 18063
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORGARD
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = KING PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 120MG
----
Product id = 19988
Application Number = 18063
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORGARD
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = KING PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 160MG
----
Product id = 19984
Application Number = 18063
Date of Application = 28,, Oct, 1986
RX/OTC/DISCN = RX
Tradename = CORGARD
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = KING PHARMS
ProductNo = 005
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 20019
Application Number = 18647
Date of Application = 25,, May, 1983
RX/OTC/DISCN = RX
Tradename = CORZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = KING PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;40MG
----
Product id = 20020
Application Number = 18647
Date of Application = 25,, May, 1983
RX/OTC/DISCN = RX
Tradename = CORZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = KING PHARMS
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 5MG;80MG
----
Product id = 25160
Application Number = 74172
Date of Application = 31,, Oct, 1993
RX/OTC/DISCN = RX
Tradename = NADOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 25161
Application Number = 74172
Date of Application = 31,, Oct, 1993
RX/OTC/DISCN = RX
Tradename = NADOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 25162
Application Number = 74172
Date of Application = 31,, Oct, 1993
RX/OTC/DISCN = RX
Tradename = NADOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 25155
Application Number = 74229
Date of Application = 30,, Aug, 1996
RX/OTC/DISCN = RX
Tradename = NADOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 25156
Application Number = 74229
Date of Application = 30,, Aug, 1996
RX/OTC/DISCN = RX
Tradename = NADOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 25157
Application Number = 74255
Date of Application = 24,, Jan, 1996
RX/OTC/DISCN = RX
Tradename = NADOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 25158
Application Number = 74255
Date of Application = 24,, Jan, 1996
RX/OTC/DISCN = DISCN
Tradename = NADOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 120MG
----
Product id = 25159
Application Number = 74255
Date of Application = 24,, Jan, 1996
RX/OTC/DISCN = DISCN
Tradename = NADOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 160MG
----
Product id = 25166
Application Number = 74368
Date of Application = 31,, Aug, 1994
RX/OTC/DISCN = DISCN
Tradename = NADOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 25167
Application Number = 74368
Date of Application = 31,, Aug, 1994
RX/OTC/DISCN = DISCN
Tradename = NADOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 120MG
----
Product id = 25168
Application Number = 74368
Date of Application = 31,, Aug, 1994
RX/OTC/DISCN = DISCN
Tradename = NADOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 160MG
----
Product id = 25163
Application Number = 74501
Date of Application = 9,, Nov, 1995
RX/OTC/DISCN = RX
Tradename = NADOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 25164
Application Number = 74501
Date of Application = 9,, Nov, 1995
RX/OTC/DISCN = RX
Tradename = NADOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 25165
Application Number = 74501
Date of Application = 9,, Nov, 1995
RX/OTC/DISCN = RX
Tradename = NADOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 25169
Application Number = 77833
Date of Application = 30,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = NADOLOL AND BENDROFLUMETHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;40MG
----
Product id = 25170
Application Number = 77833
Date of Application = 30,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = NADOLOL AND BENDROFLUMETHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG;80MG
----
Product id = 25171
Application Number = 78688
Date of Application = 15,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = NADOLOL AND BENDROFLUMETHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;40MG
----
Product id = 25172
Application Number = 78688
Date of Application = 15,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = NADOLOL AND BENDROFLUMETHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG;80MG
----