nalbuphine-hydrochloride
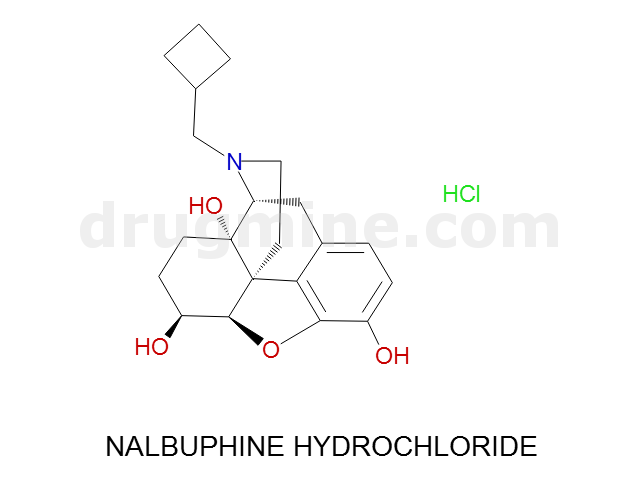
Name: NALBUPHINE HYDROCHLORIDE
ID :
MW: 357
Number of atoms: 26
Molecular_Formula: C21H27NO4
Alogp: 2.119
Indication class : Antagonist (to narcotics); Analgesic
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
nalbuphine hydrochloride containing products summary
There are in total 18 different products containing the active ingredient nalbuphine hydrochloride. From the 18 drug products, 14 have been discontinued.Product id = 9741
Application Number = 18024
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NUBAIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ENDO PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 9742
Application Number = 18024
Date of Application = 27,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = NUBAIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ENDO PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG/ML
----
Product id = 9517
Application Number = 20200
Date of Application = 12,, Mar, 1993
RX/OTC/DISCN = DISCN
Tradename = NALBUPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 001
Tecode =
Rld = No
Strength = 1.5MG/ML
----
Product id = 9514
Application Number = 70751
Date of Application = 2,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = NALBUPHINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 9515
Application Number = 70752
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = NALBUPHINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/ML
----
Product id = 9526
Application Number = 70914
Date of Application = 3,, Feb, 1989
RX/OTC/DISCN = RX
Tradename = NALBUPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 10MG/ML
----
Product id = 9527
Application Number = 70915
Date of Application = 3,, Feb, 1989
RX/OTC/DISCN = RX
Tradename = NALBUPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 10MG/ML
----
Product id = 9528
Application Number = 70916
Date of Application = 3,, Feb, 1989
RX/OTC/DISCN = RX
Tradename = NALBUPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 20MG/ML
----
Product id = 9516
Application Number = 70917
Date of Application = 3,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = NALBUPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/ML
----
Product id = 9529
Application Number = 70918
Date of Application = 3,, Feb, 1989
RX/OTC/DISCN = RX
Tradename = NALBUPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 20MG/ML
----
Product id = 9518
Application Number = 72070
Date of Application = 10,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALBUPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 9519
Application Number = 72071
Date of Application = 10,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALBUPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 9520
Application Number = 72072
Date of Application = 10,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALBUPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 9521
Application Number = 72073
Date of Application = 10,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALBUPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/ML
----
Product id = 9522
Application Number = 72074
Date of Application = 10,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALBUPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/ML
----
Product id = 9523
Application Number = 72075
Date of Application = 10,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALBUPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/ML
----
Product id = 9524
Application Number = 74471
Date of Application = 19,, Mar, 1998
RX/OTC/DISCN = DISCN
Tradename = NALBUPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 9525
Application Number = 74471
Date of Application = 19,, Mar, 1998
RX/OTC/DISCN = DISCN
Tradename = NALBUPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BARR
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG/ML
----