nalidixic-acid
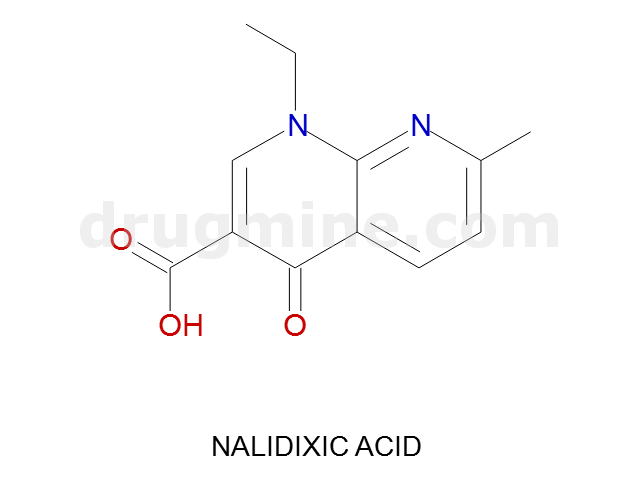

Name: NALIDIXIC ACID
ID :
MW: 232
Number of atoms: 17
Molecular_Formula: C12H12N2O3
Alogp: 1.176
Indication class : Antibacterial
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
nalidixic acid containing products summary
There are in total 10 different products containing the active ingredient nalidixic acid. From the 10 drug products, 10 have been discontinued.Product id = 25389
Application Number = 14214
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NEGGRAM
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 002
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25390
Application Number = 14214
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NEGGRAM
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 004
Tecode =
Rld = No
Strength = 500MG
----
Product id = 25388
Application Number = 14214
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NEGGRAM
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 005
Tecode =
Rld = No
Strength = 1GM
----
Product id = 14751
Application Number = 17430
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NEGGRAM
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG/5ML
----
Product id = 25175
Application Number = 70270
Date of Application = 29,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = NALIDIXIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25176
Application Number = 70271
Date of Application = 29,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = NALIDIXIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 25174
Application Number = 70272
Date of Application = 29,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = NALIDIXIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 1GM
----
Product id = 25177
Application Number = 71919
Date of Application = 29,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = NALIDIXIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 1GM
----
Product id = 25178
Application Number = 71936
Date of Application = 29,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = NALIDIXIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25179
Application Number = 72061
Date of Application = 29,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = NALIDIXIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----