naproxen
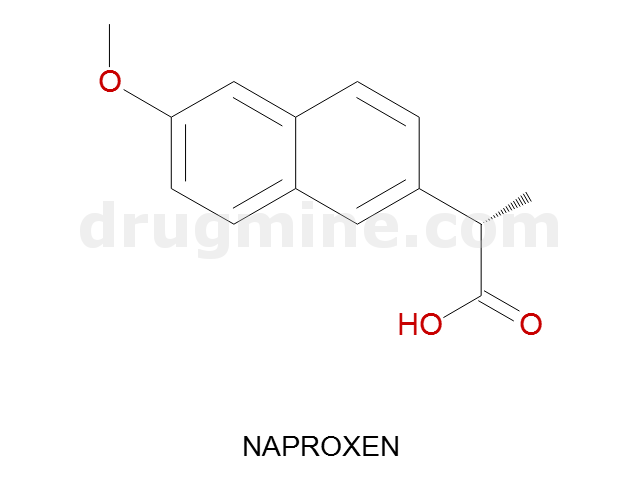

Name: NAPROXEN
ID :
MW: 230
Number of atoms: 17
Molecular_Formula: C14H14O3
Alogp: 2.849
Indication class : Anti-Inflammatory; Antipyretic; Analgesic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
naproxen containing products summary
There are in total 95 different products containing the active ingredient naproxen. From the 95 drug products, 41 have been discontinued.Product id = 25193
Application Number = 17581
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = NAPROSYN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROCHE PALO
ProductNo = 002
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 25194
Application Number = 17581
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = NAPROSYN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROCHE PALO
ProductNo = 003
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 25195
Application Number = 17581
Date of Application = 15,, Apr, 1982
RX/OTC/DISCN = RX
Tradename = NAPROSYN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROCHE PALO
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = 500MG
----
Product id = 14749
Application Number = 18965
Date of Application = 23,, Mar, 1987
RX/OTC/DISCN = RX
Tradename = NAPROSYN
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = ROCHE PALO
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 25MG/ML
----
Product id = 15516
Application Number = 20067
Date of Application = 14,, Oct, 1994
RX/OTC/DISCN = RX
Tradename = EC-NAPROSYN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = N
Applicant Name = ROCHE PALO
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 375MG
----
Product id = 15517
Application Number = 20067
Date of Application = 14,, Oct, 1994
RX/OTC/DISCN = RX
Tradename = EC-NAPROSYN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = N
Applicant Name = ROCHE PALO
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 500MG
----
Product id = 113
Application Number = 21507
Date of Application = 14,, Nov, 2003
RX/OTC/DISCN = DISCN
Tradename = PREVACID NAPRAPAC 250 (COPACKAGED)
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS, TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS NA
ProductNo = 002
Tecode =
Rld = No
Strength = 15MG,N/A;N/A,250MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 114
Application Number = 21507
Date of Application = 14,, Nov, 2003
RX/OTC/DISCN = DISCN
Tradename = PREVACID NAPRAPAC 375 (COPACKAGED)
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS, TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS NA
ProductNo = 003
Tecode =
Rld = No
Strength = 15MG,N/A;N/A,375MG
----
Product id = 115
Application Number = 21507
Date of Application = 14,, Nov, 2003
RX/OTC/DISCN = DISCN
Tradename = PREVACID NAPRAPAC 500 (COPACKAGED)
Route/format = ORAL / CAPSULE, DELAYED REL PELLETS, TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS NA
ProductNo = 004
Tecode =
Rld = No
Strength = 15MG,N/A;N/A,500MG
----
Product id = 15610
Application Number = 22511
Date of Application = 30,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = VIMOVO
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = N
Applicant Name = HORIZON PHARMA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 20MG BASE;500MG
----
Product id = 15609
Application Number = 22511
Date of Application = 30,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = VIMOVO
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = N
Applicant Name = HORIZON PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE;375MG
----
Product id = 25202
Application Number = 74105
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25204
Application Number = 74105
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 375MG
----
Product id = 25206
Application Number = 74105
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG
----
Product id = 25211
Application Number = 74110
Date of Application = 30,, Oct, 1992
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HAMILTON PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25212
Application Number = 74110
Date of Application = 30,, Oct, 1992
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HAMILTON PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 375MG
----
Product id = 25213
Application Number = 74110
Date of Application = 30,, Oct, 1992
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HAMILTON PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG
----
Product id = 25220
Application Number = 74111
Date of Application = 28,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25221
Application Number = 74111
Date of Application = 28,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 375MG
----
Product id = 25222
Application Number = 74111
Date of Application = 28,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG
----
Product id = 25226
Application Number = 74121
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 25227
Application Number = 74121
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 25228
Application Number = 74121
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 25244
Application Number = 74129
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25247
Application Number = 74129
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 375MG
----
Product id = 25250
Application Number = 74129
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG
----
Product id = 25241
Application Number = 74140
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 25242
Application Number = 74140
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 25243
Application Number = 74140
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 25256
Application Number = 74163
Date of Application = 10,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25258
Application Number = 74163
Date of Application = 10,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 375MG
----
Product id = 25260
Application Number = 74163
Date of Application = 10,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG
----
Product id = 25232
Application Number = 74182
Date of Application = 27,, Jun, 1996
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25233
Application Number = 74182
Date of Application = 27,, Jun, 1996
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 002
Tecode =
Rld = No
Strength = 375MG
----
Product id = 25234
Application Number = 74182
Date of Application = 27,, Jun, 1996
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG
----
Product id = 14750
Application Number = 74190
Date of Application = 30,, Mar, 1994
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG/ML
----
Product id = 25245
Application Number = 74201
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 25248
Application Number = 74201
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 25251
Application Number = 74201
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 25253
Application Number = 74207
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25254
Application Number = 74207
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 375MG
----
Product id = 25255
Application Number = 74207
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG
----
Product id = 25238
Application Number = 74211
Date of Application = 28,, Feb, 1994
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25239
Application Number = 74211
Date of Application = 28,, Feb, 1994
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode =
Rld = No
Strength = 375MG
----
Product id = 25240
Application Number = 74211
Date of Application = 28,, Feb, 1994
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG
----
Product id = 25246
Application Number = 74216
Date of Application = 11,, Apr, 1996
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25249
Application Number = 74216
Date of Application = 11,, Apr, 1996
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 375MG
----
Product id = 25252
Application Number = 74216
Date of Application = 11,, Apr, 1996
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG
----
Product id = 25235
Application Number = 74263
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25236
Application Number = 74263
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 375MG
----
Product id = 25237
Application Number = 74263
Date of Application = 21,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG
----
Product id = 25203
Application Number = 74410
Date of Application = 28,, Apr, 1995
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25205
Application Number = 74410
Date of Application = 28,, Apr, 1995
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 375MG
----
Product id = 25207
Application Number = 74410
Date of Application = 28,, Apr, 1995
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG
----
Product id = 25257
Application Number = 74457
Date of Application = 31,, May, 1995
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25259
Application Number = 74457
Date of Application = 31,, May, 1995
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 375MG
----
Product id = 25261
Application Number = 74457
Date of Application = 31,, May, 1995
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG
----
Product id = 15532
Application Number = 74936
Date of Application = 24,, Feb, 1998
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 375MG
----
Product id = 15533
Application Number = 74936
Date of Application = 24,, Feb, 1998
RX/OTC/DISCN = DISCN
Tradename = NAPROXEN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG
----
Product id = 15540
Application Number = 75061
Date of Application = 18,, Feb, 1998
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 15541
Application Number = 75061
Date of Application = 18,, Feb, 1998
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 15542
Application Number = 75227
Date of Application = 30,, Jun, 1998
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 15543
Application Number = 75227
Date of Application = 30,, Jun, 1998
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 15538
Application Number = 75337
Date of Application = 26,, May, 1999
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 15539
Application Number = 75337
Date of Application = 26,, May, 1999
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = PLIVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 15536
Application Number = 75390
Date of Application = 19,, Apr, 2001
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 15537
Application Number = 75390
Date of Application = 19,, Apr, 2001
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 25196
Application Number = 75927
Date of Application = 18,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 25197
Application Number = 75927
Date of Application = 18,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 25198
Application Number = 75927
Date of Application = 18,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 25214
Application Number = 76494
Date of Application = 14,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 25215
Application Number = 76494
Date of Application = 14,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 25216
Application Number = 76494
Date of Application = 14,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 25229
Application Number = 77339
Date of Application = 27,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO R AND D
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 25230
Application Number = 77339
Date of Application = 27,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO R AND D
ProductNo = 002
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 25231
Application Number = 77339
Date of Application = 27,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO R AND D
ProductNo = 003
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 25208
Application Number = 78250
Date of Application = 28,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 25209
Application Number = 78250
Date of Application = 28,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 25210
Application Number = 78250
Date of Application = 28,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 25262
Application Number = 78620
Date of Application = 7,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 25263
Application Number = 78620
Date of Application = 7,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 25264
Application Number = 78620
Date of Application = 7,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 25217
Application Number = 91305
Date of Application = 24,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 25218
Application Number = 91305
Date of Application = 24,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 25219
Application Number = 91305
Date of Application = 24,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 25223
Application Number = 91416
Date of Application = 14,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MARKSANS PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 25224
Application Number = 91416
Date of Application = 14,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MARKSANS PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 25225
Application Number = 91416
Date of Application = 14,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MARKSANS PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 15534
Application Number = 91432
Date of Application = 19,, Sep, 2011
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 15535
Application Number = 91432
Date of Application = 19,, Sep, 2011
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 25199
Application Number = 200429
Date of Application = 8,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 25200
Application Number = 200429
Date of Application = 8,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 25201
Application Number = 200429
Date of Application = 8,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = NAPROXEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA USA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 15544
Application Number = 202461
Date of Application = 27,, Sep, 2013
RX/OTC/DISCN = RX
Tradename = NAPROXEN AND ESOMEPRAZOLE MAGNESIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE;375MG
----
Product id = 15545
Application Number = 202461
Date of Application = 27,, Sep, 2013
RX/OTC/DISCN = RX
Tradename = NAPROXEN AND ESOMEPRAZOLE MAGNESIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE;500MG
----