nizatidine
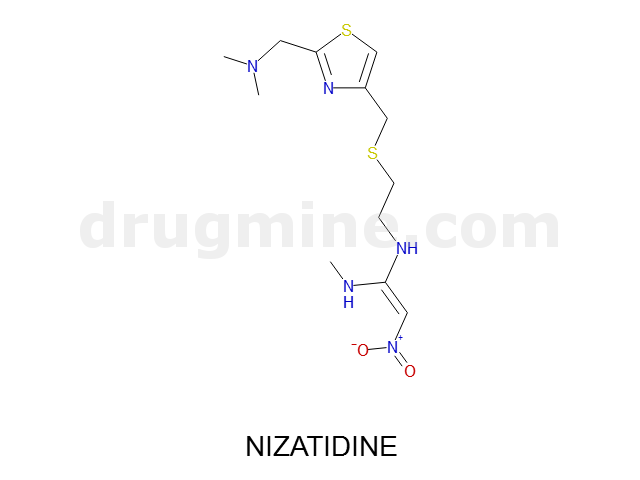

Name: NIZATIDINE
ID :
MW: 331
Number of atoms: 21
Molecular_Formula: C12H21N5O2S2
Alogp: 0.643
Indication class : Anti-Ulcerative
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
nizatidine containing products summary
There are in total 23 different products containing the active ingredient nizatidine. From the 23 drug products, 8 have been discontinued.Product id = 1117
Application Number = 19508
Date of Application = 12,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = AXID
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = SMITHKLINE BEECHAM
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG
----
Product id = 1118
Application Number = 19508
Date of Application = 12,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = AXID
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = SMITHKLINE BEECHAM
ProductNo = 002
Tecode =
Rld = No
Strength = 300MG
----
Product id = 18241
Application Number = 20555
Date of Application = 9,, May, 1996
RX/OTC/DISCN = OTC
Tradename = AXID AR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = 75MG
----
Product id = 13743
Application Number = 21494
Date of Application = 25,, May, 2004
RX/OTC/DISCN = RX
Tradename = AXID
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = BRAINTREE
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 15MG/ML
----
Product id = 2625
Application Number = 75461
Date of Application = 8,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG
----
Product id = 2627
Application Number = 75461
Date of Application = 8,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 300MG
----
Product id = 2641
Application Number = 75616
Date of Application = 9,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 2642
Application Number = 75616
Date of Application = 9,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 2626
Application Number = 75668
Date of Application = 12,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 2628
Application Number = 75668
Date of Application = 12,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 2635
Application Number = 75806
Date of Application = 5,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 2637
Application Number = 75806
Date of Application = 5,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 300MG
----
Product id = 2636
Application Number = 75934
Date of Application = 9,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG
----
Product id = 2638
Application Number = 75934
Date of Application = 9,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 300MG
----
Product id = 2639
Application Number = 76178
Date of Application = 5,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 2640
Application Number = 76178
Date of Application = 5,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 2629
Application Number = 76383
Date of Application = 23,, Jan, 2003
RX/OTC/DISCN = DISCN
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG
----
Product id = 2630
Application Number = 76383
Date of Application = 23,, Jan, 2003
RX/OTC/DISCN = DISCN
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode =
Rld = No
Strength = 300MG
----
Product id = 2631
Application Number = 77314
Date of Application = 15,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 2632
Application Number = 77314
Date of Application = 15,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 13956
Application Number = 90576
Date of Application = 18,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = NIZATIDINE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 001
Tecode = AA
Rld = No
Strength = 15MG/ML
----
Product id = 2633
Application Number = 90618
Date of Application = 15,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 2634
Application Number = 90618
Date of Application = 15,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = NIZATIDINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 300MG
----