ondansetron-hydrochloride
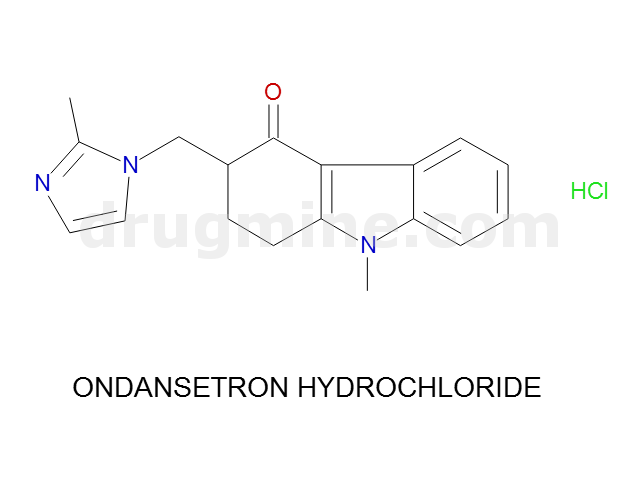


Name: ONDANSETRON HYDROCHLORIDE
ID :
MW: 293
Number of atoms: 22
Molecular_Formula: C18H19N3O
Alogp: 2.634
Indication class : Anti-Anxiety Agent; Anti-Emetic; Antischizophrenic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
ondansetron hydrochloride containing products summary
There are in total 102 different products containing the active ingredient ondansetron hydrochloride. From the 102 drug products, 26 have been discontinued.Product id = 11486
Application Number = 20007
Date of Application = 4,, Jan, 1991
RX/OTC/DISCN = RX
Tradename = ZOFRAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 2MG BASE/ML
----
Product id = 11488
Application Number = 20007
Date of Application = 10,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = ZOFRAN PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = EQ 2MG BASE/ML
----
Product id = 29802
Application Number = 20103
Date of Application = 31,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = ZOFRAN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 29803
Application Number = 20103
Date of Application = 31,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = ZOFRAN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 29804
Application Number = 20103
Date of Application = 27,, Aug, 1999
RX/OTC/DISCN = RX
Tradename = ZOFRAN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = EQ 24MG BASE
----
Product id = 11487
Application Number = 20403
Date of Application = 31,, Jan, 1995
RX/OTC/DISCN = DISCN
Tradename = ZOFRAN AND DEXTROSE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.64MG BASE/ML
----
Product id = 14066
Application Number = 20605
Date of Application = 24,, Jan, 1997
RX/OTC/DISCN = RX
Tradename = ZOFRAN
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = EQ 4MG BASE/5ML
----
Product id = 9850
Application Number = 21915
Date of Application = 27,, Dec, 2006
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE AND SODIUM CHLORIDE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 0.64MG BASE/ML
----
Product id = 25610
Application Number = 76183
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 24MG BASE
----
Product id = 25608
Application Number = 76183
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 25607
Application Number = 76183
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 25609
Application Number = 76183
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 16MG BASE
----
Product id = 25638
Application Number = 76252
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 25639
Application Number = 76252
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 25640
Application Number = 76252
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 24MG BASE
----
Product id = 9831
Application Number = 76695
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9861
Application Number = 76696
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9868
Application Number = 76759
Date of Application = 22,, Nov, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9859
Application Number = 76780
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9829
Application Number = 76781
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9843
Application Number = 76876
Date of Application = 22,, Nov, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 25619
Application Number = 76930
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 25620
Application Number = 76930
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 25621
Application Number = 76930
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 24MG BASE
----
Product id = 13967
Application Number = 76960
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 4MG BASE/5ML
----
Product id = 9823
Application Number = 76967
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9858
Application Number = 76972
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9826
Application Number = 76974
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9847
Application Number = 76978
Date of Application = 26,, Feb, 2007
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE AND DEXTROSE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.64MG BASE/ML
----
Product id = 13969
Application Number = 77009
Date of Application = 30,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 4MG BASE/5ML
----
Product id = 9855
Application Number = 77011
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 25633
Application Number = 77050
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS (IN)
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 25634
Application Number = 77050
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS (IN)
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 25627
Application Number = 77112
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 25628
Application Number = 77112
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 25629
Application Number = 77112
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 24MG BASE
----
Product id = 9842
Application Number = 77172
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SUN PHARM INDS (IN)
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9866
Application Number = 77173
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 25624
Application Number = 77303
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 25625
Application Number = 77303
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 8MG BASE
----
Product id = 25626
Application Number = 77303
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 24MG BASE
----
Product id = 25602
Application Number = 77306
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 25603
Application Number = 77306
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 9852
Application Number = 77343
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9848
Application Number = 77348
Date of Application = 1,, Feb, 2007
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE AND DEXTROSE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.64MG BASE/ML
----
Product id = 9830
Application Number = 77365
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9821
Application Number = 77368
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9863
Application Number = 77387
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9841
Application Number = 77430
Date of Application = 27,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9832
Application Number = 77473
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9849
Application Number = 77480
Date of Application = 22,, Nov, 2006
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE AND DEXTROSE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.64MG BASE/ML
----
Product id = 25630
Application Number = 77517
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 25631
Application Number = 77517
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 25632
Application Number = 77517
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 24MG BASE
----
Product id = 25611
Application Number = 77535
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 25612
Application Number = 77535
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 25613
Application Number = 77535
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 24MG BASE
----
Product id = 9860
Application Number = 77541
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9838
Application Number = 77544
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 25614
Application Number = 77545
Date of Application = 6,, Sep, 2007
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 25615
Application Number = 77545
Date of Application = 6,, Sep, 2007
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 8MG BASE
----
Product id = 25616
Application Number = 77545
Date of Application = 6,, Sep, 2007
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 24MG BASE
----
Product id = 9862
Application Number = 77548
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9865
Application Number = 77551
Date of Application = 27,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9844
Application Number = 77577
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9836
Application Number = 77582
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9869
Application Number = 77716
Date of Application = 26,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 25635
Application Number = 77729
Date of Application = 28,, Mar, 2011
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 25636
Application Number = 77729
Date of Application = 28,, Mar, 2011
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 8MG BASE
----
Product id = 25637
Application Number = 77729
Date of Application = 28,, Mar, 2011
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 24MG BASE
----
Product id = 9833
Application Number = 77840
Date of Application = 19,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 25622
Application Number = 77851
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NATCO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 25623
Application Number = 77851
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NATCO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 9867
Application Number = 78014
Date of Application = 21,, Mar, 2008
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TARO PHARMS IRELAND
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 13965
Application Number = 78127
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 4MG BASE/5ML
----
Product id = 9840
Application Number = 78180
Date of Application = 26,, Mar, 2007
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9851
Application Number = 78244
Date of Application = 23,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9820
Application Number = 78257
Date of Application = 23,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9856
Application Number = 78287
Date of Application = 22,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CLARIS LIFESCIENCES
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9824
Application Number = 78288
Date of Application = 22,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CLARIS LIFESCIENCES
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9845
Application Number = 78291
Date of Application = 13,, Apr, 2009
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE AND DEXTROSE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.64MG BASE/ML
----
Product id = 9846
Application Number = 78308
Date of Application = 17,, Mar, 2008
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE AND DEXTROSE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CLARIS LIFESCIENCES
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.64MG BASE/ML
----
Product id = 25604
Application Number = 78539
Date of Application = 31,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 25605
Application Number = 78539
Date of Application = 31,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 25606
Application Number = 78539
Date of Application = 31,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 24MG BASE
----
Product id = 13966
Application Number = 78776
Date of Application = 28,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 4MG BASE/5ML
----
Product id = 9857
Application Number = 78945
Date of Application = 3,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EMCURE PHARMS LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9864
Application Number = 79032
Date of Application = 18,, Nov, 2008
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9837
Application Number = 79039
Date of Application = 18,, Nov, 2008
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9827
Application Number = 79224
Date of Application = 25,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAND PHARMA LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9834
Application Number = 90116
Date of Application = 14,, Apr, 2010
RX/OTC/DISCN = DISCN
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9825
Application Number = 90424
Date of Application = 16,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EMCURE PHARMS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9828
Application Number = 90648
Date of Application = 15,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAND PHARMA LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9835
Application Number = 90883
Date of Application = 5,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 13968
Application Number = 91342
Date of Application = 27,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = SILARX
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 4MG BASE/5ML
----
Product id = 13964
Application Number = 91483
Date of Application = 31,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 4MG BASE/5ML
----
Product id = 9854
Application Number = 202253
Date of Application = 19,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BD RX
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9822
Application Number = 202599
Date of Application = 21,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9853
Application Number = 202600
Date of Application = 21,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 9839
Application Number = 203711
Date of Application = 8,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = QILU PHARM CO LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 2MG BASE/ML
----
Product id = 25617
Application Number = 203761
Date of Application = 23,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 25618
Application Number = 203761
Date of Application = 23,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = ONDANSETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----