oxacillin-sodium
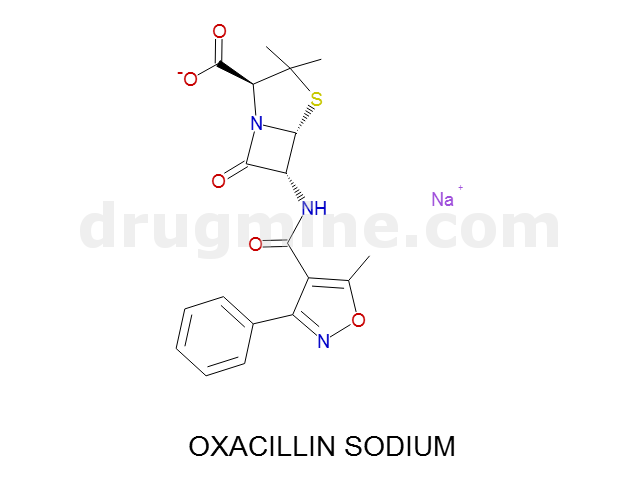
Name: OXACILLIN SODIUM
ID :
MW: 400
Number of atoms: 28
Molecular_Formula: C19H18N3O5S
Alogp: 0.161
Indication class : Antibacterial
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
oxacillin sodium containing products summary
There are in total 59 different products containing the active ingredient oxacillin sodium. From the 59 drug products, 44 have been discontinued.Product id = 3080
Application Number = 50118
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROSTAPHLIN
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 4929
Application Number = 50194
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROSTAPHLIN
Route/format = ORAL / FOR SOLUTION
Application Type = N
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/5ML
----
Product id = 9920
Application Number = 50195
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 9921
Application Number = 50195
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 9917
Application Number = 50195
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = APOTHECON
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9918
Application Number = 50195
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = APOTHECON
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9919
Application Number = 50195
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = APOTHECON
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 4GM BASE/VIAL
----
Product id = 6095
Application Number = 50640
Date of Application = 26,, Oct, 1989
RX/OTC/DISCN = RX
Tradename = BACTOCILL IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 20MG BASE/ML
----
Product id = 6096
Application Number = 50640
Date of Application = 26,, Oct, 1989
RX/OTC/DISCN = RX
Tradename = BACTOCILL IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 40MG BASE/ML
----
Product id = 6088
Application Number = 61334
Date of Application = 26,, Mar, 1982
RX/OTC/DISCN = DISCN
Tradename = BACTOCILL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 006
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6090
Application Number = 61334
Date of Application = 26,, Mar, 1982
RX/OTC/DISCN = DISCN
Tradename = BACTOCILL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 007
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6092
Application Number = 61334
Date of Application = 26,, Mar, 1982
RX/OTC/DISCN = DISCN
Tradename = BACTOCILL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 008
Tecode =
Rld = No
Strength = EQ 4GM BASE/VIAL
----
Product id = 6094
Application Number = 61334
Date of Application = 26,, Mar, 1982
RX/OTC/DISCN = DISCN
Tradename = BACTOCILL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 009
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6093
Application Number = 61334
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BACTOCILL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 010
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 1120
Application Number = 61336
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BACTOCILL
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE
----
Product id = 1122
Application Number = 61336
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BACTOCILL
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 2723
Application Number = 61450
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 2722
Application Number = 61450
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 250MG BASE
----
Product id = 4878
Application Number = 61457
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = ORAL / FOR SOLUTION
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/5ML
----
Product id = 9942
Application Number = 61490
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 9943
Application Number = 61490
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 9939
Application Number = 61490
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = EQ 1GM BASE/VIAL
----
Product id = 9940
Application Number = 61490
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode = AP
Rld = Yes
Strength = EQ 2GM BASE/VIAL
----
Product id = 9941
Application Number = 61490
Date of Application = 9,, May, 1991
RX/OTC/DISCN = RX
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 006
Tecode = AP
Rld = Yes
Strength = EQ 10GM BASE/VIAL
----
Product id = 2724
Application Number = 62222
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE
----
Product id = 2725
Application Number = 62222
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 1121
Application Number = 62241
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BACTOCILL
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE
----
Product id = 1123
Application Number = 62241
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BACTOCILL
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 4879
Application Number = 62252
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = ORAL / FOR SOLUTION
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/5ML
----
Product id = 4830
Application Number = 62321
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BACTOCILL
Route/format = ORAL / FOR SOLUTION
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/5ML
----
Product id = 9929
Application Number = 62711
Date of Application = 3,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ELKINS SINN
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 9930
Application Number = 62711
Date of Application = 3,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ELKINS SINN
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 9925
Application Number = 62711
Date of Application = 3,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ELKINS SINN
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9926
Application Number = 62711
Date of Application = 3,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ELKINS SINN
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9927
Application Number = 62711
Date of Application = 3,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ELKINS SINN
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 4GM BASE/VIAL
----
Product id = 9928
Application Number = 62711
Date of Application = 3,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ELKINS SINN
ProductNo = 006
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6089
Application Number = 62736
Date of Application = 19,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = BACTOCILL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6091
Application Number = 62736
Date of Application = 19,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = BACTOCILL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 12714
Application Number = 62737
Date of Application = 23,, Dec, 1986
RX/OTC/DISCN = RX
Tradename = OXACILLIN SODIUM
Route/format = INTRAVENOUS / POWDER
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 1GM BASE/VIAL
----
Product id = 12715
Application Number = 62737
Date of Application = 23,, Dec, 1986
RX/OTC/DISCN = RX
Tradename = OXACILLIN SODIUM
Route/format = INTRAVENOUS / POWDER
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 2GM BASE/VIAL
----
Product id = 9931
Application Number = 62798
Date of Application = 11,, Dec, 1995
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ISTITUTO BIO ITA SPA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9932
Application Number = 62798
Date of Application = 11,, Dec, 1995
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ISTITUTO BIO ITA SPA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9933
Application Number = 62798
Date of Application = 11,, Dec, 1995
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ISTITUTO BIO ITA SPA
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 125MG BASE/VIAL
----
Product id = 9934
Application Number = 62798
Date of Application = 11,, Dec, 1995
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ISTITUTO BIO ITA SPA
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 9935
Application Number = 62798
Date of Application = 11,, Dec, 1995
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ISTITUTO BIO ITA SPA
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 9948
Application Number = 62856
Date of Application = 26,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 9949
Application Number = 62856
Date of Application = 26,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 9944
Application Number = 62856
Date of Application = 26,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9945
Application Number = 62856
Date of Application = 26,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9946
Application Number = 62856
Date of Application = 26,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 4GM BASE/VIAL
----
Product id = 9947
Application Number = 62984
Date of Application = 29,, Sep, 1988
RX/OTC/DISCN = DISCN
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 9938
Application Number = 91245
Date of Application = 30,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT PHARMS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 9936
Application Number = 91246
Date of Application = 30,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT PHARMS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9937
Application Number = 91246
Date of Application = 30,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT PHARMS
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9915
Application Number = 91486
Date of Application = 25,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9916
Application Number = 91486
Date of Application = 25,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9924
Application Number = 201538
Date of Application = 18,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 9922
Application Number = 201539
Date of Application = 18,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9923
Application Number = 201539
Date of Application = 18,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = OXACILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----