pemoline
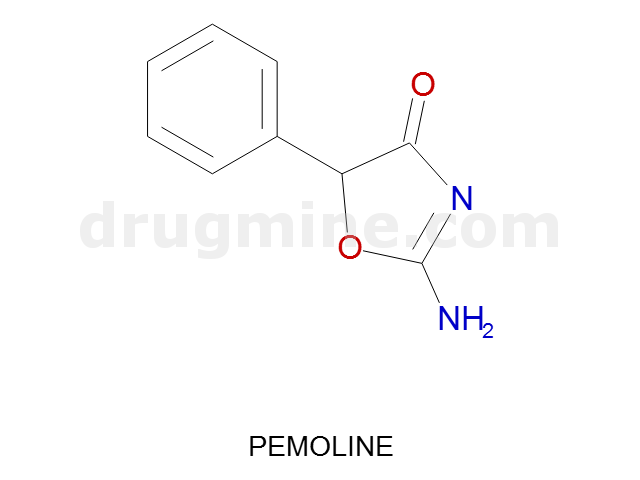
Name: PEMOLINE
ID :
MW: 176
Number of atoms: 13
Molecular_Formula: C9H8N2O2
Alogp: 1.26
Indication class : Stimulant (central)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
pemoline containing products summary
There are in total 24 different products containing the active ingredient pemoline. From the 24 drug products, 24 have been discontinued.Product id = 20082
Application Number = 16832
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CYLERT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 18.75MG
----
Product id = 20083
Application Number = 16832
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CYLERT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBOTT
ProductNo = 002
Tecode =
Rld = No
Strength = 37.5MG
----
Product id = 20084
Application Number = 16832
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CYLERT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBOTT
ProductNo = 003
Tecode =
Rld = No
Strength = 75MG
----
Product id = 15247
Application Number = 17703
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CYLERT
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 37.5MG
----
Product id = 25971
Application Number = 75030
Date of Application = 29,, Jan, 1999
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 37.5MG
----
Product id = 25972
Application Number = 75030
Date of Application = 29,, Jan, 1999
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 75MG
----
Product id = 25970
Application Number = 75030
Date of Application = 22,, Feb, 2000
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 18.75MG
----
Product id = 25967
Application Number = 75286
Date of Application = 27,, Dec, 1999
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 18.75MG
----
Product id = 25968
Application Number = 75286
Date of Application = 30,, Jun, 1999
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 37.5MG
----
Product id = 25969
Application Number = 75286
Date of Application = 30,, Jun, 1999
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 75MG
----
Product id = 25976
Application Number = 75287
Date of Application = 13,, Jun, 2001
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 18.75MG
----
Product id = 25977
Application Number = 75287
Date of Application = 18,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 37.5MG
----
Product id = 25978
Application Number = 75287
Date of Application = 18,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 75MG
----
Product id = 25973
Application Number = 75328
Date of Application = 19,, Apr, 2000
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 18.75MG
----
Product id = 25974
Application Number = 75328
Date of Application = 19,, Apr, 2000
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 37.5MG
----
Product id = 25975
Application Number = 75328
Date of Application = 19,, Apr, 2000
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 75MG
----
Product id = 15348
Application Number = 75555
Date of Application = 18,, Feb, 2000
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 37.5MG
----
Product id = 25961
Application Number = 75595
Date of Application = 28,, Feb, 2000
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 18.75MG
----
Product id = 25962
Application Number = 75595
Date of Application = 28,, Feb, 2000
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode =
Rld = No
Strength = 37.5MG
----
Product id = 25963
Application Number = 75595
Date of Application = 28,, Feb, 2000
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 003
Tecode =
Rld = No
Strength = 75MG
----
Product id = 15347
Application Number = 75678
Date of Application = 26,, Jul, 2000
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 37.5MG
----
Product id = 25966
Application Number = 75726
Date of Application = 30,, Mar, 2001
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 001
Tecode =
Rld = No
Strength = 75MG
----
Product id = 25965
Application Number = 75726
Date of Application = 30,, Mar, 2001
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 002
Tecode =
Rld = No
Strength = 37.5MG
----
Product id = 25964
Application Number = 75726
Date of Application = 30,, Mar, 2001
RX/OTC/DISCN = DISCN
Tradename = PEMOLINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 003
Tecode =
Rld = No
Strength = 18.75MG
----