pioglitazone-hydrochloride
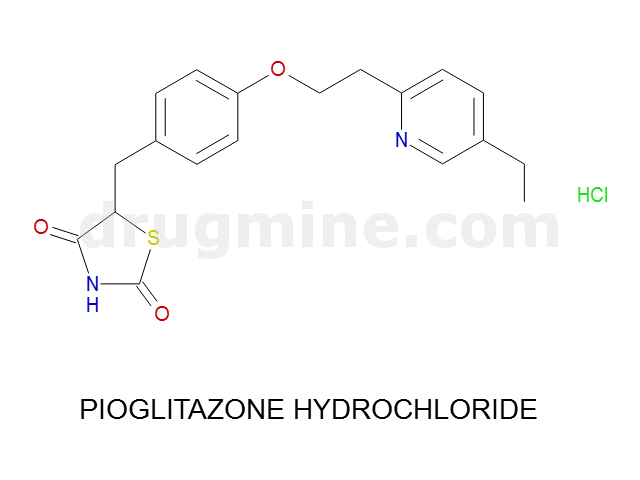

Name: PIOGLITAZONE HYDROCHLORIDE
ID :
MW: 356
Number of atoms: 25
Molecular_Formula: C19H20N2O3S
Alogp: 3.907
Indication class : Antidiabetic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
pioglitazone hydrochloride containing products summary
There are in total 63 different products containing the active ingredient pioglitazone hydrochloride. From the 63 drug products, zero have been discontinued.Product id = 17331
Application Number = 21073
Date of Application = 15,, Jul, 1999
RX/OTC/DISCN = RX
Tradename = ACTOS
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 15MG BASE
----
Product id = 17332
Application Number = 21073
Date of Application = 15,, Jul, 1999
RX/OTC/DISCN = RX
Tradename = ACTOS
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 17333
Application Number = 21073
Date of Application = 15,, Jul, 1999
RX/OTC/DISCN = RX
Tradename = ACTOS
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = EQ 45MG BASE
----
Product id = 17329
Application Number = 21842
Date of Application = 29,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = ACTOPLUS MET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 15MG BASE
----
Product id = 17330
Application Number = 21842
Date of Application = 29,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = ACTOPLUS MET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 850MG;EQ 15MG BASE
----
Product id = 20760
Application Number = 21925
Date of Application = 28,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = DUETACT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 2MG;30MG
----
Product id = 20761
Application Number = 21925
Date of Application = 28,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = DUETACT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 4MG;30MG
----
Product id = 15628
Application Number = 22024
Date of Application = 12,, May, 2009
RX/OTC/DISCN = RX
Tradename = ACTOPLUS MET XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = 1GM;EQ 15MG BASE
----
Product id = 15629
Application Number = 22024
Date of Application = 12,, May, 2009
RX/OTC/DISCN = RX
Tradename = ACTOPLUS MET XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 002
Tecode =
Rld = Yes
Strength = 1GM;EQ 30MG BASE
----
Product id = 25685
Application Number = 22426
Date of Application = 25,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = OSENI
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 25MG BASE;EQ 15MG BASE
----
Product id = 25686
Application Number = 22426
Date of Application = 25,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = OSENI
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 25MG BASE;EQ 30MG BASE
----
Product id = 25687
Application Number = 22426
Date of Application = 25,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = OSENI
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 003
Tecode =
Rld = Yes
Strength = EQ 25MG BASE;EQ 45MG BASE
----
Product id = 25682
Application Number = 22426
Date of Application = 25,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = OSENI
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 12.5MG BASE;EQ 15MG BASE
----
Product id = 25683
Application Number = 22426
Date of Application = 25,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = OSENI
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 12.5MG BASE;EQ 30MG BASE
----
Product id = 25684
Application Number = 22426
Date of Application = 25,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = OSENI
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 006
Tecode =
Rld = No
Strength = EQ 12.5MG BASE;EQ 45MG BASE
----
Product id = 26290
Application Number = 76798
Date of Application = 26,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 15MG BASE
----
Product id = 26291
Application Number = 76798
Date of Application = 26,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 26292
Application Number = 76798
Date of Application = 26,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 45MG BASE
----
Product id = 26278
Application Number = 76801
Date of Application = 17,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 15MG BASE
----
Product id = 26279
Application Number = 76801
Date of Application = 17,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 26280
Application Number = 76801
Date of Application = 17,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 45MG BASE
----
Product id = 26284
Application Number = 77210
Date of Application = 10,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 15MG BASE
----
Product id = 26285
Application Number = 77210
Date of Application = 10,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 26286
Application Number = 77210
Date of Application = 10,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 45MG BASE
----
Product id = 26269
Application Number = 78383
Date of Application = 12,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 15MG BASE
----
Product id = 26270
Application Number = 78383
Date of Application = 12,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 26271
Application Number = 78383
Date of Application = 12,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 45MG BASE
----
Product id = 26266
Application Number = 78472
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BRECKENRIDGE PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 15MG BASE
----
Product id = 26267
Application Number = 78472
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BRECKENRIDGE PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 26268
Application Number = 78472
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BRECKENRIDGE PHARM
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 45MG BASE
----
Product id = 26281
Application Number = 78670
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 15MG BASE
----
Product id = 26282
Application Number = 78670
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 26283
Application Number = 78670
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 45MG BASE
----
Product id = 26300
Application Number = 90406
Date of Application = 25,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 15MG BASE
----
Product id = 26301
Application Number = 90406
Date of Application = 25,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG;EQ 15MG BASE
----
Product id = 26304
Application Number = 91155
Date of Application = 10,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 15MG BASE
----
Product id = 26305
Application Number = 91155
Date of Application = 10,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG;EQ 15MG BASE
----
Product id = 26302
Application Number = 91273
Date of Application = 16,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 15MG BASE
----
Product id = 26303
Application Number = 91273
Date of Application = 16,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG;EQ 15MG BASE
----
Product id = 26287
Application Number = 91298
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 15MG BASE
----
Product id = 26288
Application Number = 91298
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 26289
Application Number = 91298
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 45MG BASE
----
Product id = 26260
Application Number = 200044
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 15MG BASE
----
Product id = 26261
Application Number = 200044
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 26262
Application Number = 200044
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 45MG BASE
----
Product id = 26263
Application Number = 200268
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 15MG BASE
----
Product id = 26264
Application Number = 200268
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 26265
Application Number = 200268
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 45MG BASE
----
Product id = 26298
Application Number = 200823
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 15MG BASE
----
Product id = 26299
Application Number = 200823
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG;EQ 15MG BASE
----
Product id = 26296
Application Number = 201049
Date of Application = 4,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG;30MG
----
Product id = 26297
Application Number = 201049
Date of Application = 4,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND GLIMEPIRIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 4MG;30MG
----
Product id = 26306
Application Number = 202001
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 15MG BASE
----
Product id = 26307
Application Number = 202001
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG;EQ 15MG BASE
----
Product id = 26293
Application Number = 202456
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 15MG BASE
----
Product id = 26294
Application Number = 202456
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 26295
Application Number = 202456
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 45MG BASE
----
Product id = 26275
Application Number = 202467
Date of Application = 6,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 15MG BASE
----
Product id = 26276
Application Number = 202467
Date of Application = 6,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 26277
Application Number = 202467
Date of Application = 6,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 45MG BASE
----
Product id = 26272
Application Number = 204133
Date of Application = 7,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 15MG BASE
----
Product id = 26273
Application Number = 204133
Date of Application = 7,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 26274
Application Number = 204133
Date of Application = 7,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 45MG BASE
----