prilocaine
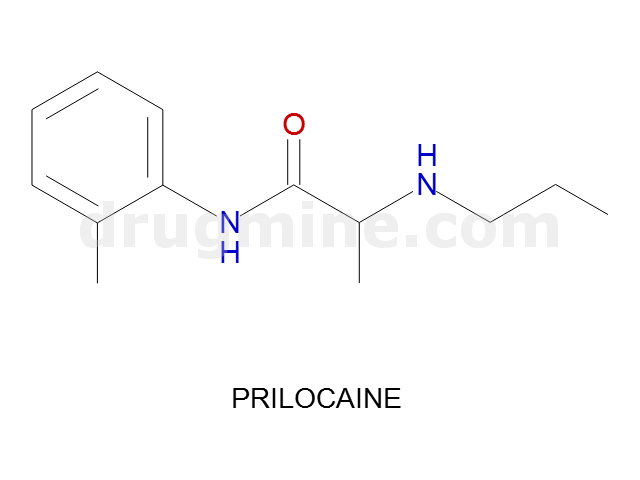


Name: PRILOCAINE
ID :
MW: 220
Number of atoms: 16
Molecular_Formula: C13H20N2O
Alogp: 2.263
Indication class : Anesthetic (local)
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
prilocaine containing products summary
There are in total 6 different products containing the active ingredient prilocaine. From the 6 drug products, 1 have been discontinued.Product id = 4138
Application Number = 19941
Date of Application = 30,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = EMLA
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = ACTAVIS LABS UT INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 2.5%;2.5%
----
Product id = 4513
Application Number = 20962
Date of Application = 4,, Feb, 1998
RX/OTC/DISCN = DISCN
Tradename = EMLA
Route/format = TOPICAL / DISC
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5%;2.5%
----
Product id = 5353
Application Number = 21451
Date of Application = 19,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = ORAQIX
Route/format = PERIODONTAL / GEL
Application Type = N
Applicant Name = DENTSPLY PHARM
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2.5%;2.5%
----
Product id = 4290
Application Number = 76290
Date of Application = 25,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = LIDOCAINE AND PRILOCAINE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = HI TECH PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5%;2.5%
----
Product id = 4291
Application Number = 76320
Date of Application = 27,, Aug, 2003
RX/OTC/DISCN = RX
Tradename = LIDOCAINE AND PRILOCAINE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TOLMAR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5%;2.5%
----
Product id = 4289
Application Number = 76453
Date of Application = 18,, Aug, 2003
RX/OTC/DISCN = RX
Tradename = LIDOCAINE AND PRILOCAINE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5%;2.5%
----