propranolol-hydrochloride
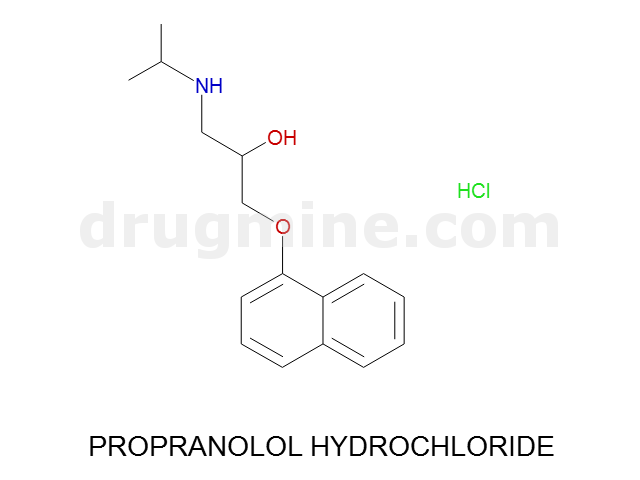
Name: PROPRANOLOL HYDROCHLORIDE
ID :
MW: 259
Number of atoms: 19
Molecular_Formula: C16H21NO2
Alogp: 2.54
Indication class : Cardiac Depressant (anti-arrhythmic); Anti-Adrenergic (beta-receptor)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
propranolol hydrochloride containing products summary
There are in total 199 different products containing the active ingredient propranolol hydrochloride. From the 199 drug products, 127 have been discontinued.Product id = 22919
Application Number = 16418
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = INDERAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 22921
Application Number = 16418
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = INDERAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 40MG
----
Product id = 22920
Application Number = 16418
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = INDERAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 003
Tecode =
Rld = No
Strength = 20MG
----
Product id = 22923
Application Number = 16418
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = INDERAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 004
Tecode =
Rld = No
Strength = 80MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 22922
Application Number = 16418
Date of Application = 18,, Oct, 1982
RX/OTC/DISCN = DISCN
Tradename = INDERAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 009
Tecode =
Rld = No
Strength = 60MG
----
Product id = 22924
Application Number = 16418
Date of Application = 18,, Oct, 1982
RX/OTC/DISCN = DISCN
Tradename = INDERAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 010
Tecode =
Rld = No
Strength = 90MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 10555
Application Number = 16419
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE CORP
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 1MG/ML
----
Product id = 22925
Application Number = 18031
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = INDERIDE-40/25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 25MG;40MG
----
Product id = 22926
Application Number = 18031
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = INDERIDE-80/25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 549
Application Number = 18553
Date of Application = 19,, Apr, 1983
RX/OTC/DISCN = RX
Tradename = INDERAL LA
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = CRANFORD PHARMS LLC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 160MG
----
Product id = 547
Application Number = 18553
Date of Application = 19,, Apr, 1983
RX/OTC/DISCN = RX
Tradename = INDERAL LA
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = CRANFORD PHARMS LLC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 548
Application Number = 18553
Date of Application = 19,, Apr, 1983
RX/OTC/DISCN = RX
Tradename = INDERAL LA
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = CRANFORD PHARMS LLC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 120MG
----
Product id = 546
Application Number = 18553
Date of Application = 18,, Mar, 1987
RX/OTC/DISCN = RX
Tradename = INDERAL LA
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = CRANFORD PHARMS LLC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 552
Application Number = 19059
Date of Application = 3,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = INDERIDE LA 80/50
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;80MG
----
Product id = 550
Application Number = 19059
Date of Application = 3,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = INDERIDE LA 120/50
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG;120MG
----
Product id = 551
Application Number = 19059
Date of Application = 3,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = INDERIDE LA 160/50
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG;160MG
----
Product id = 14724
Application Number = 19536
Date of Application = 12,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = INDERAL
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 563
Application Number = 21438
Date of Application = 12,, Mar, 2003
RX/OTC/DISCN = RX
Tradename = INNOPRAN XL
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 001
Tecode = BX
Rld = No
Strength = 80MG
----
Product id = 564
Application Number = 21438
Date of Application = 12,, Mar, 2003
RX/OTC/DISCN = RX
Tradename = INNOPRAN XL
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 002
Tecode = BX
Rld = No
Strength = 120MG
----
Product id = 26919
Application Number = 70103
Date of Application = 22,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26961
Application Number = 70120
Date of Application = 6,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26962
Application Number = 70121
Date of Application = 6,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26963
Application Number = 70122
Date of Application = 6,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26964
Application Number = 70123
Date of Application = 29,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 26965
Application Number = 70124
Date of Application = 6,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 26887
Application Number = 70125
Date of Application = 30,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26888
Application Number = 70126
Date of Application = 30,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26889
Application Number = 70127
Date of Application = 30,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26891
Application Number = 70128
Date of Application = 30,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 10560
Application Number = 70135
Date of Application = 15,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SMITH AND NEPHEW
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 10562
Application Number = 70136
Date of Application = 15,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SOLOPAK
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 10561
Application Number = 70137
Date of Application = 15,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SMITH AND NEPHEW
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 26983
Application Number = 70140
Date of Application = 30,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26987
Application Number = 70141
Date of Application = 30,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26991
Application Number = 70142
Date of Application = 30,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26995
Application Number = 70143
Date of Application = 15,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 26999
Application Number = 70144
Date of Application = 30,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 26984
Application Number = 70175
Date of Application = 13,, May, 1986
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26988
Application Number = 70176
Date of Application = 13,, May, 1986
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26992
Application Number = 70177
Date of Application = 13,, May, 1986
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 27000
Application Number = 70178
Date of Application = 13,, May, 1986
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 26924
Application Number = 70213
Date of Application = 19,, Nov, 1985
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26922
Application Number = 70213
Date of Application = 19,, Nov, 1985
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26923
Application Number = 70213
Date of Application = 19,, Nov, 1985
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26927
Application Number = 70213
Date of Application = 19,, Nov, 1985
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 26925
Application Number = 70213
Date of Application = 8,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 005
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 26973
Application Number = 70217
Date of Application = 1,, Aug, 1986
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26974
Application Number = 70218
Date of Application = 1,, Aug, 1986
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26975
Application Number = 70219
Date of Application = 1,, Aug, 1986
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26976
Application Number = 70220
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 26977
Application Number = 70221
Date of Application = 14,, Apr, 1986
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 26970
Application Number = 70232
Date of Application = 7,, Oct, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26971
Application Number = 70233
Date of Application = 23,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26972
Application Number = 70234
Date of Application = 23,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 27021
Application Number = 70301
Date of Application = 18,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;40MG
----
Product id = 27023
Application Number = 70305
Date of Application = 18,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 26893
Application Number = 70306
Date of Application = 9,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26894
Application Number = 70307
Date of Application = 9,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26895
Application Number = 70308
Date of Application = 9,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26896
Application Number = 70309
Date of Application = 1,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 26897
Application Number = 70310
Date of Application = 9,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 26917
Application Number = 70319
Date of Application = 22,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26918
Application Number = 70320
Date of Application = 22,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26920
Application Number = 70321
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 26921
Application Number = 70322
Date of Application = 4,, Aug, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 26985
Application Number = 70378
Date of Application = 19,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26989
Application Number = 70379
Date of Application = 19,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26993
Application Number = 70380
Date of Application = 19,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26996
Application Number = 70381
Date of Application = 19,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 27001
Application Number = 70382
Date of Application = 19,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 26978
Application Number = 70438
Date of Application = 15,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26979
Application Number = 70439
Date of Application = 15,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26980
Application Number = 70440
Date of Application = 15,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26981
Application Number = 70441
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 26982
Application Number = 70442
Date of Application = 15,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 26944
Application Number = 70516
Date of Application = 7,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26945
Application Number = 70517
Date of Application = 7,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26946
Application Number = 70518
Date of Application = 7,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26947
Application Number = 70519
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 26948
Application Number = 70520
Date of Application = 7,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 26949
Application Number = 70521
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 90MG
----
Product id = 26986
Application Number = 70548
Date of Application = 10,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26990
Application Number = 70549
Date of Application = 11,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26994
Application Number = 70550
Date of Application = 11,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 27002
Application Number = 70551
Date of Application = 10,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 26950
Application Number = 70663
Date of Application = 13,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26952
Application Number = 70664
Date of Application = 13,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26954
Application Number = 70665
Date of Application = 13,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26956
Application Number = 70666
Date of Application = 10,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 26958
Application Number = 70667
Date of Application = 13,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 14013
Application Number = 70690
Date of Application = 15,, May, 1987
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = Yes
Strength = 40MG/5ML
----
Product id = 27009
Application Number = 70704
Date of Application = 1,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;40MG
----
Product id = 27010
Application Number = 70705
Date of Application = 1,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 26943
Application Number = 70757
Date of Application = 3,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 26939
Application Number = 70814
Date of Application = 3,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26940
Application Number = 70815
Date of Application = 3,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26941
Application Number = 70816
Date of Application = 3,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26942
Application Number = 70817
Date of Application = 3,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 27007
Application Number = 70851
Date of Application = 15,, May, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;40MG
----
Product id = 27008
Application Number = 70852
Date of Application = 15,, May, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 27014
Application Number = 70947
Date of Application = 1,, Apr, 1987
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 25MG;80MG
----
Product id = 27013
Application Number = 70947
Date of Application = 4,, Mar, 1987
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;40MG
----
Product id = 14012
Application Number = 70979
Date of Application = 15,, May, 1987
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = Yes
Strength = 20MG/5ML
----
Product id = 27017
Application Number = 71060
Date of Application = 26,, Aug, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;40MG
----
Product id = 27018
Application Number = 71061
Date of Application = 26,, Aug, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 26997
Application Number = 71098
Date of Application = 6,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 27005
Application Number = 71126
Date of Application = 2,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE & HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;40MG
----
Product id = 27006
Application Number = 71127
Date of Application = 2,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE & HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 27003
Application Number = 71183
Date of Application = 6,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 90MG
----
Product id = 26933
Application Number = 71288
Date of Application = 22,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 90MG
----
Product id = 26898
Application Number = 71327
Date of Application = 1,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 90MG
----
Product id = 26899
Application Number = 71368
Date of Application = 5,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INTERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26900
Application Number = 71369
Date of Application = 5,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INTERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26901
Application Number = 71370
Date of Application = 5,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INTERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26902
Application Number = 71371
Date of Application = 5,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INTERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 3963
Application Number = 71388
Date of Application = 15,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE INTENSOL
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/ML
----
Product id = 26890
Application Number = 71495
Date of Application = 31,, Dec, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 26892
Application Number = 71496
Date of Application = 31,, Dec, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 90MG
----
Product id = 27022
Application Number = 71498
Date of Application = 18,, Dec, 1991
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;40MG
----
Product id = 27024
Application Number = 71501
Date of Application = 18,, Dec, 1991
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 26966
Application Number = 71515
Date of Application = 8,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26967
Application Number = 71516
Date of Application = 8,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26968
Application Number = 71517
Date of Application = 8,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26969
Application Number = 71518
Date of Application = 8,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 27011
Application Number = 71552
Date of Application = 1,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;40MG
----
Product id = 27012
Application Number = 71553
Date of Application = 1,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 26951
Application Number = 71658
Date of Application = 5,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26953
Application Number = 71687
Date of Application = 5,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26955
Application Number = 71688
Date of Application = 5,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26959
Application Number = 71689
Date of Application = 5,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 27019
Application Number = 71771
Date of Application = 26,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;40MG
----
Product id = 27020
Application Number = 71772
Date of Application = 26,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 26998
Application Number = 71791
Date of Application = 15,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 27004
Application Number = 71792
Date of Application = 15,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 90MG
----
Product id = 26934
Application Number = 71972
Date of Application = 6,, Apr, 1988
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26935
Application Number = 71973
Date of Application = 6,, Apr, 1988
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26936
Application Number = 71974
Date of Application = 6,, Apr, 1988
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26937
Application Number = 71975
Date of Application = 6,, Apr, 1988
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 26938
Application Number = 71976
Date of Application = 6,, Apr, 1988
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 80MG
----
Product id = 26886
Application Number = 71977
Date of Application = 6,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 90MG
----
Product id = 14010
Application Number = 71984
Date of Application = 3,, Mar, 1989
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = MORTON GROVE
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/5ML
----
Product id = 14011
Application Number = 71985
Date of Application = 3,, Mar, 1989
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = MORTON GROVE
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG/5ML
----
Product id = 27015
Application Number = 72042
Date of Application = 14,, Mar, 1988
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;40MG
----
Product id = 27016
Application Number = 72043
Date of Application = 14,, Mar, 1988
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;80MG
----
Product id = 26908
Application Number = 72063
Date of Application = 29,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26909
Application Number = 72066
Date of Application = 29,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26910
Application Number = 72067
Date of Application = 29,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26911
Application Number = 72068
Date of Application = 29,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 26912
Application Number = 72069
Date of Application = 29,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 26913
Application Number = 72117
Date of Application = 23,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26914
Application Number = 72118
Date of Application = 23,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26915
Application Number = 72119
Date of Application = 23,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26916
Application Number = 72120
Date of Application = 23,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 26957
Application Number = 72197
Date of Application = 5,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 26960
Application Number = 72198
Date of Application = 5,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 90MG
----
Product id = 26926
Application Number = 72275
Date of Application = 9,, Jun, 1989
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 694
Application Number = 72499
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = INWOOD LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 695
Application Number = 72500
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = INWOOD LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG
----
Product id = 696
Application Number = 72501
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = INWOOD LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 120MG
----
Product id = 697
Application Number = 72502
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = INWOOD LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 160MG
----
Product id = 10556
Application Number = 75792
Date of Application = 29,, Aug, 2000
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 10557
Application Number = 75826
Date of Application = 31,, Aug, 2001
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 10559
Application Number = 76400
Date of Application = 26,, Feb, 2003
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 10558
Application Number = 77760
Date of Application = 31,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 698
Application Number = 78022
Date of Application = 15,, Feb, 2007
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 699
Application Number = 78022
Date of Application = 15,, Feb, 2007
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 700
Application Number = 78022
Date of Application = 15,, Feb, 2007
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 120MG
----
Product id = 701
Application Number = 78022
Date of Application = 15,, Feb, 2007
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = 160MG
----
Product id = 702
Application Number = 78065
Date of Application = 26,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = NORTEC DEV ASSOC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 703
Application Number = 78065
Date of Application = 26,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = NORTEC DEV ASSOC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 704
Application Number = 78065
Date of Application = 26,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = NORTEC DEV ASSOC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 120MG
----
Product id = 705
Application Number = 78065
Date of Application = 26,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = NORTEC DEV ASSOC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 160MG
----
Product id = 26928
Application Number = 78213
Date of Application = 10,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NORTHSTAR HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26929
Application Number = 78213
Date of Application = 10,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NORTHSTAR HLTHCARE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26930
Application Number = 78213
Date of Application = 10,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NORTHSTAR HLTHCARE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26931
Application Number = 78213
Date of Application = 10,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NORTHSTAR HLTHCARE
ProductNo = 004
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 26932
Application Number = 78213
Date of Application = 10,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NORTHSTAR HLTHCARE
ProductNo = 005
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 706
Application Number = 78311
Date of Application = 6,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = UPSHER SMITH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 707
Application Number = 78311
Date of Application = 6,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = UPSHER SMITH
ProductNo = 002
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 708
Application Number = 78311
Date of Application = 6,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = UPSHER SMITH
ProductNo = 003
Tecode = AB
Rld = No
Strength = 120MG
----
Product id = 709
Application Number = 78311
Date of Application = 6,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = UPSHER SMITH
ProductNo = 004
Tecode = AB
Rld = No
Strength = 160MG
----
Product id = 686
Application Number = 78494
Date of Application = 10,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 687
Application Number = 78494
Date of Application = 10,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 688
Application Number = 78494
Date of Application = 10,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 003
Tecode = AB
Rld = No
Strength = 120MG
----
Product id = 689
Application Number = 78494
Date of Application = 10,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 004
Tecode = AB
Rld = No
Strength = 160MG
----
Product id = 690
Application Number = 78703
Date of Application = 15,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = APTALIS PHARMATECH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 691
Application Number = 78703
Date of Application = 15,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = APTALIS PHARMATECH
ProductNo = 002
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 692
Application Number = 78703
Date of Application = 15,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = APTALIS PHARMATECH
ProductNo = 003
Tecode = AB
Rld = No
Strength = 120MG
----
Product id = 693
Application Number = 78703
Date of Application = 15,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = APTALIS PHARMATECH
ProductNo = 004
Tecode = AB
Rld = No
Strength = 160MG
----
Product id = 26903
Application Number = 78955
Date of Application = 2,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26904
Application Number = 78955
Date of Application = 2,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26905
Application Number = 78955
Date of Application = 2,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26906
Application Number = 78955
Date of Application = 2,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 26907
Application Number = 78955
Date of Application = 2,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 710
Application Number = 90321
Date of Application = 25,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 711
Application Number = 90321
Date of Application = 25,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 712
Application Number = 90321
Date of Application = 25,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 120MG
----
Product id = 713
Application Number = 90321
Date of Application = 25,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 160MG
----
Product id = 13828
Application Number = 205410
Date of Application = 14,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = HEMANGEOL
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = PIERRE FABRE DERMA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 4.28MG/ML
----