pseudoephedrine-hydrochloride
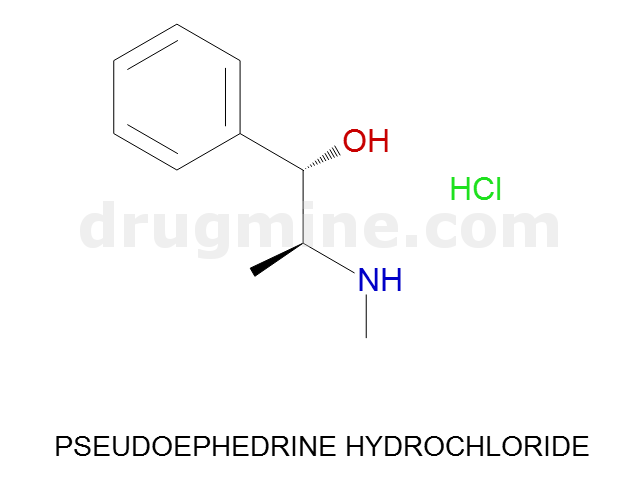


Name: PSEUDOEPHEDRINE HYDROCHLORIDE
ID :
MW: 165
Number of atoms: 12
Molecular_Formula: C10H15NO
Alogp: 1.234
Indication class : Adrenergic (vasoconstrictor)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
pseudoephedrine hydrochloride containing products summary
There are in total 75 different products containing the active ingredient pseudoephedrine hydrochloride. From the 75 drug products, 33 have been discontinued.Product id = 14876
Application Number = 12575
Date of Application = 4,, Apr, 1984
RX/OTC/DISCN = DISCN
Tradename = ACTIFED W/ CODEINE
Route/format = ORAL / SYRUP
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG/5ML;30MG/5ML;1.25MG/5ML
----
Product id = 650
Application Number = 17603
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NOVAFED
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 120MG
----
Product id = 758
Application Number = 17941
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SUDAFED 12 HOUR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode =
Rld = No
Strength = 120MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 567
Application Number = 18747
Date of Application = 6,, Mar, 1986
RX/OTC/DISCN = DISCN
Tradename = ISOCLOR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = FISONS
ProductNo = 001
Tecode =
Rld = No
Strength = 8MG;120MG
----
Product id = 716
Application Number = 18843
Date of Application = 18,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = PSEUDOEPHEDRINE HYDROCHLORIDE AND CHLORPHENIRAMINE MALEATE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = GRAHAM DM
ProductNo = 001
Tecode =
Rld = No
Strength = 12MG;120MG
----
Product id = 715
Application Number = 18844
Date of Application = 20,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = PSEUDOEPHEDRINE HYDROCHLORIDE AND CHLORPHENIRAMINE MALEATE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = GRAHAM DM
ProductNo = 001
Tecode =
Rld = No
Strength = 8MG;120MG
----
Product id = 368
Application Number = 18935
Date of Application = 15,, Apr, 1985
RX/OTC/DISCN = DISCN
Tradename = CODIMAL-L.A. 12
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = SCHWARZ PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 12MG;120MG
----
Product id = 300
Application Number = 18996
Date of Application = 17,, Jun, 1985
RX/OTC/DISCN = DISCN
Tradename = ACTIFED
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = 120MG;5MG
----
Product id = 13745
Application Number = 19014
Date of Application = 11,, Jun, 1985
RX/OTC/DISCN = DISCN
Tradename = BENYLIN
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG/5ML;30MG/5ML
----
Product id = 14985
Application Number = 19279
Date of Application = 24,, Aug, 1984
RX/OTC/DISCN = DISCN
Tradename = DIMETANE-DX
Route/format = ORAL / SYRUP
Application Type = N
Applicant Name = ROBINS AH
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG/5ML;10MG/5ML;30MG/5ML
----
Product id = 714
Application Number = 19428
Date of Application = 2,, Aug, 1988
RX/OTC/DISCN = DISCN
Tradename = PSEUDOEPHEDRINE HYDROCHLORIDE AND CHLORPHENIRAMINE MALEATE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = CENT PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 8MG;120MG
----
Product id = 15907
Application Number = 19672
Date of Application = 29,, Mar, 1996
RX/OTC/DISCN = DISCN
Tradename = EFIDAC 24 PSEUDOEPHEDRINE HYDROCHLORIDE/BROMPHENIRAMINE MALEATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ALZA
ProductNo = 001
Tecode =
Rld = No
Strength = 16MG;240MG
----
Product id = 17389
Application Number = 19771
Date of Application = 19,, Sep, 1989
RX/OTC/DISCN = OTC
Tradename = ADVIL COLD AND SINUS
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = 200MG;30MG
----
Product id = 3263
Application Number = 19806
Date of Application = 25,, Mar, 1994
RX/OTC/DISCN = RX
Tradename = SEMPREX-D
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = AUXILIUM PHARMS LLC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 8MG;60MG
----
Product id = 28070
Application Number = 19899
Date of Application = 31,, Dec, 1992
RX/OTC/DISCN = OTC
Tradename = SINE-AID IB
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MCNEIL CONS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG;30MG
----
Product id = 16643
Application Number = 20021
Date of Application = 15,, Dec, 1992
RX/OTC/DISCN = OTC
Tradename = SUDAFED 24 HOUR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = MCNEIL CONS
ProductNo = 002
Tecode =
Rld = Yes
Strength = 240MG
----
Product id = 15651
Application Number = 20786
Date of Application = 24,, Jan, 2011
RX/OTC/DISCN = OTC
Tradename = ALLEGRA-D 12 HOUR ALLERGY AND CONGESTION
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 002
Tecode =
Rld = Yes
Strength = 60MG;120MG
----
Product id = 15642
Application Number = 21076
Date of Application = 29,, Nov, 1999
RX/OTC/DISCN = OTC
Tradename = ALEVE-D SINUS & COLD
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = BAYER
ProductNo = 001
Tecode =
Rld = Yes
Strength = 200MG;120MG
----
Product id = 28485
Application Number = 21082
Date of Application = 1,, Mar, 2001
RX/OTC/DISCN = DISCN
Tradename = TAVIST ALLERGY/SINUS/HEADACHE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG;EQ 0.25MG BASE;30MG
----
Product id = 14665
Application Number = 21128
Date of Application = 1,, Aug, 2000
RX/OTC/DISCN = OTC
Tradename = CHILDREN'S MOTRIN COLD
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = MCNEIL CONS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 100MG/5ML;15MG/5ML
----
Product id = 16810
Application Number = 21150
Date of Application = 9,, Nov, 2007
RX/OTC/DISCN = OTC
Tradename = ZYRTEC-D 12 HOUR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = MCNEIL
ProductNo = 002
Tecode =
Rld = Yes
Strength = 5MG;120MG
----
Product id = 14656
Application Number = 21373
Date of Application = 18,, Apr, 2002
RX/OTC/DISCN = OTC
Tradename = CHILDREN'S ADVIL COLD
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/5ML;15MG/5ML
----
Product id = 938
Application Number = 21374
Date of Application = 30,, May, 2002
RX/OTC/DISCN = OTC
Tradename = ADVIL COLD AND SINUS
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 200MG FREE ACID AND POTASSIUM SALT;30MG
----
Product id = 17388
Application Number = 21441
Date of Application = 19,, Dec, 2002
RX/OTC/DISCN = OTC
Tradename = ADVIL ALLERGY SINUS
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2MG;200MG;30MG
----
Product id = 16321
Application Number = 21585
Date of Application = 22,, Jun, 2004
RX/OTC/DISCN = OTC
Tradename = MUCINEX D
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG;60MG
----
Product id = 16320
Application Number = 21585
Date of Application = 22,, Jun, 2004
RX/OTC/DISCN = OTC
Tradename = MUCINEX D
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 002
Tecode =
Rld = Yes
Strength = 1.2GM;120MG
----
Product id = 14655
Application Number = 21587
Date of Application = 24,, Feb, 2004
RX/OTC/DISCN = OTC
Tradename = CHILDREN'S ADVIL ALLERGY SINUS
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = 1MG/5ML;100MG/5ML;15MG/5ML
----
Product id = 15652
Application Number = 21704
Date of Application = 24,, Jan, 2011
RX/OTC/DISCN = OTC
Tradename = ALLEGRA-D 24 HOUR ALLERGY AND CONGESTION
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 002
Tecode =
Rld = Yes
Strength = 180MG;240MG
----
Product id = 14067
Application Number = 22439
Date of Application = 8,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = ZUTRIPRO
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = CYPRESS PHARM
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 4MG/5ML;5MG/5ML;60MG/5ML
----
Product id = 14019
Application Number = 22442
Date of Application = 8,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = REZIRA
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = CYPRESS PHARM
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 5MG/5ML;60MG/5ML
----
Product id = 717
Application Number = 71455
Date of Application = 1,, Mar, 1989
RX/OTC/DISCN = DISCN
Tradename = PSEUDOEPHEDRINE HYDROCHLORIDE AND CHLORPHENIRAMINE MALEATE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = KV PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 12MG;120MG
----
Product id = 807
Application Number = 71798
Date of Application = 16,, Mar, 1989
RX/OTC/DISCN = DISCN
Tradename = TRIPROLIDINE AND PSEUDOEPHEDRINE HYDROCHLORIDES
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = KV PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 120MG;5MG
----
Product id = 16732
Application Number = 72758
Date of Application = 25,, Nov, 1991
RX/OTC/DISCN = DISCN
Tradename = TRIPROLIDINE AND PSEUDOEPHEDRINE HYDROCHLORIDES
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = KV PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 120MG;5MG
----
Product id = 16642
Application Number = 73585
Date of Application = 31,, Oct, 1991
RX/OTC/DISCN = OTC
Tradename = SUDAFED 12 HOUR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MCNEIL CONS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 120MG
----
Product id = 22845
Application Number = 74567
Date of Application = 17,, Apr, 2001
RX/OTC/DISCN = OTC
Tradename = IBUPROHM COLD AND SINUS
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = OHM LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG;30MG
----
Product id = 16552
Application Number = 75153
Date of Application = 26,, Feb, 1999
RX/OTC/DISCN = OTC
Tradename = PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 120MG
----
Product id = 22841
Application Number = 75588
Date of Application = 8,, Apr, 2002
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CONTRACT PHARMACAL
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG;30MG
----
Product id = 15957
Application Number = 76236
Date of Application = 14,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = FEXOFENADINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG;120MG
----
Product id = 15960
Application Number = 76298
Date of Application = 12,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = FEXOFENADINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = IMPAX PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG;120MG
----
Product id = 14717
Application Number = 76478
Date of Application = 5,, Nov, 2003
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/5ML;15MG/5ML
----
Product id = 16332
Application Number = 76518
Date of Application = 17,, Mar, 2004
RX/OTC/DISCN = OTC
Tradename = NAPROXEN SODIUM AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 200MG BASE;120MG
----
Product id = 15958
Application Number = 76667
Date of Application = 18,, Nov, 2014
RX/OTC/DISCN = OTC
Tradename = FEXOFENADINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG;120MG
----
Product id = 15805
Application Number = 77170
Date of Application = 25,, Feb, 2008
RX/OTC/DISCN = OTC
Tradename = CETIRIZINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG;120MG
----
Product id = 16331
Application Number = 77381
Date of Application = 27,, Sep, 2006
RX/OTC/DISCN = OTC
Tradename = NAPROXEN SODIUM AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 220MG BASE;120MG
----
Product id = 16553
Application Number = 77442
Date of Application = 28,, Sep, 2005
RX/OTC/DISCN = OTC
Tradename = PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode =
Rld = No
Strength = 120MG
----
Product id = 22842
Application Number = 77628
Date of Application = 14,, Aug, 2006
RX/OTC/DISCN = OTC
Tradename = IBUPROFEN AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG;30MG
----
Product id = 15806
Application Number = 77991
Date of Application = 5,, Mar, 2008
RX/OTC/DISCN = OTC
Tradename = CETIRIZINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG;120MG
----
Product id = 15959
Application Number = 79043
Date of Application = 22,, Jun, 2011
RX/OTC/DISCN = OTC
Tradename = FEXOFENADINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode =
Rld = No
Strength = 180MG;240MG
----
Product id = 29246
Application Number = 85273
Date of Application = 12,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = TRIPROLIDINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG;2.5MG
----
Product id = 15044
Application Number = 88116
Date of Application = 4,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = MYFED
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG/5ML;1.25MG/5ML
----
Product id = 29243
Application Number = 88117
Date of Application = 19,, Apr, 1983
RX/OTC/DISCN = DISCN
Tradename = TRIPROLIDINE AND PSEUDOEPHEDRINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WEST WARD
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG;2.5MG
----
Product id = 27063
Application Number = 88193
Date of Application = 17,, May, 1983
RX/OTC/DISCN = DISCN
Tradename = PSEUDOEPHEDRINE HYDROCHLORIDE AND TRIPROLIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG;2.5MG
----
Product id = 14998
Application Number = 88283
Date of Application = 20,, Apr, 1984
RX/OTC/DISCN = DISCN
Tradename = HISTAFED
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = CENCI
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG/5ML;1.25MG/5ML
----
Product id = 29242
Application Number = 88318
Date of Application = 13,, Jan, 1984
RX/OTC/DISCN = DISCN
Tradename = TRIPROLIDINE AND PSEUDOEPHEDRINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 60MG;2.5MG
----
Product id = 14874
Application Number = 88344
Date of Application = 9,, Feb, 1984
RX/OTC/DISCN = DISCN
Tradename = ACTAHIST
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = CENCI
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG/5ML;1.25MG/5ML
----
Product id = 15161
Application Number = 88474
Date of Application = 12,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = TRILITRON
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = NEWTRON PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG/5ML;1.25MG/5ML
----
Product id = 29218
Application Number = 88515
Date of Application = 9,, Jan, 1985
RX/OTC/DISCN = DISCN
Tradename = TRILITRON
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NEWTRON PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG;2.5MG
----
Product id = 29247
Application Number = 88578
Date of Application = 21,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = TRIPROLIDINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG;2.5MG
----
Product id = 19989
Application Number = 88602
Date of Application = 11,, Apr, 1985
RX/OTC/DISCN = DISCN
Tradename = CORPHED
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG;2.5MG
----
Product id = 29238
Application Number = 88630
Date of Application = 17,, May, 1984
RX/OTC/DISCN = DISCN
Tradename = TRIPHED
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG;2.5MG
----
Product id = 15159
Application Number = 88704
Date of Application = 22,, Mar, 1985
RX/OTC/DISCN = RX
Tradename = TRIACIN-C
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = STI PHARMA LLC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 10MG/5ML;30MG/5ML;1.25MG/5ML
----
Product id = 14907
Application Number = 88722
Date of Application = 7,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = BROMANATE DM
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = ALPHARMA US PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG/5ML;10MG/5ML;30MG/5ML
----
Product id = 14909
Application Number = 88811
Date of Application = 7,, Jun, 1985
RX/OTC/DISCN = RX
Tradename = BROMFED-DM
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 2MG/5ML;10MG/5ML;30MG/5ML
----
Product id = 15165
Application Number = 88833
Date of Application = 16,, Nov, 1984
RX/OTC/DISCN = DISCN
Tradename = TRIPROLIDINE HCL, PSEUDOEPHEDRINE HCL AND CODEINE PHOSPHATE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/5ML;30MG/5ML;1.25MG/5ML
----
Product id = 17497
Application Number = 88860
Date of Application = 31,, Jan, 1985
RX/OTC/DISCN = DISCN
Tradename = ALLERFED
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PVT FORM
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG;2.5MG
----
Product id = 15164
Application Number = 89018
Date of Application = 23,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = TRIPROLIDINE AND PSEUDOEPHEDRINE HYDROCHLORIDES W/ CODEINE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = CENCI
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/5ML;30MG/5ML;1.25MG/5ML
----
Product id = 14910
Application Number = 89681
Date of Application = 22,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = BROMFED-DM
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG/5ML;10MG/5ML;30MG/5ML
----
Product id = 15961
Application Number = 90818
Date of Application = 29,, Jan, 2015
RX/OTC/DISCN = OTC
Tradename = FEXOFENADINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG;120MG
----
Product id = 15807
Application Number = 90922
Date of Application = 28,, Sep, 2012
RX/OTC/DISCN = OTC
Tradename = CETIRIZINE HYDROCHLORIDE AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG;120MG
----
Product id = 14913
Application Number = 202940
Date of Application = 21,, Jul, 2014
RX/OTC/DISCN = RX
Tradename = BROMPHENIRAMINE MALEATE, PSEUDOEPHEDRINE HYDROCHLORIDE AND DEXTROMETHORPHAN HYDROBROMIDE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AA
Rld = No
Strength = 2MG/5ML;10MG/5ML;30MG/5ML
----
Product id = 13854
Application Number = 203838
Date of Application = 26,, Nov, 2014
RX/OTC/DISCN = RX
Tradename = HYDROCODONE BITARTRATE, CHLORPHENIRAMINE MALEATE AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = TRIS PHARMA INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 4MG/5ML;5MG/5ML;60MG/5ML
----
Product id = 13853
Application Number = 203839
Date of Application = 28,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = HYDROCODONE BITARTRATE AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = TRIS PHARMA INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 5MG/5ML;60MG/5ML
----
Product id = 13855
Application Number = 204627
Date of Application = 29,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = HYDROCODONE BITARTRATE, CHLORPHENIRAMINE MALEATE AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 4MG/5ML;5MG/5ML;60MG/5ML
----
Product id = 13852
Application Number = 204658
Date of Application = 29,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = HYDROCODONE BITARTRATE AND PSEUDOEPHEDRINE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 5MG/5ML;60MG/5ML
----
Product id = 14912
Application Number = 205292
Date of Application = 15,, Jul, 2014
RX/OTC/DISCN = RX
Tradename = BROMPHENIRAMINE MALEATE, PSEUDOEPHEDRINE HYDROCHLORIDE AND DEXTROMETHORPHAN HYDROBROMIDE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 2MG/5ML;10MG/5ML;30MG/5ML
----