pyrazinamide
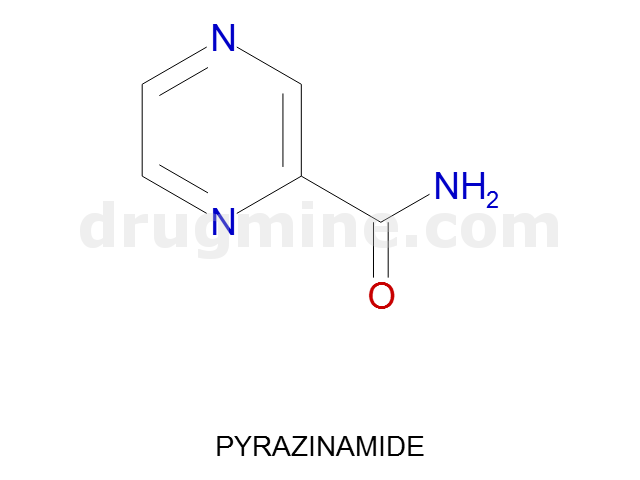

Name: PYRAZINAMIDE
ID :
MW: 123
Number of atoms: 9
Molecular_Formula: C5H5N3O
Alogp: -1.041
Indication class : Antibacterial (tuberculostatic)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
pyrazinamide containing products summary
There are in total 3 different products containing the active ingredient pyrazinamide. From the 3 drug products, zero have been discontinued.Product id = 27531
Application Number = 50705
Date of Application = 31,, May, 1994
RX/OTC/DISCN = RX
Tradename = RIFATER
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = Yes
Strength = 50MG;300MG;120MG
----
Product id = 27065
Application Number = 80157
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = PYRAZINAMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 500MG
----
Product id = 27066
Application Number = 81319
Date of Application = 30,, Jun, 1992
RX/OTC/DISCN = RX
Tradename = PYRAZINAMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MIKART
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----