reserpine

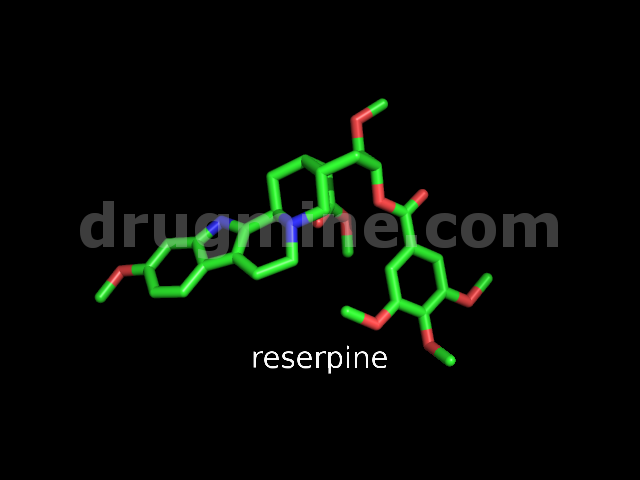
Name: RESERPINE
ID :
MW: 609
Number of atoms: 44
Molecular_Formula: C33H40N2O9
Alogp: 4.242
Indication class : Antihypertensive
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
reserpine containing products summary
There are in total 141 different products containing the active ingredient reserpine. From the 141 drug products, 139 have been discontinued.Product id = 27890
Application Number = 9115
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPASIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27891
Application Number = 9115
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPASIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 003
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27892
Application Number = 9115
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPASIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 004
Tecode =
Rld = No
Strength = 1MG
----
Product id = 4564
Application Number = 9115
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPASIL
Route/format = ORAL / ELIXIR
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 005
Tecode =
Rld = No
Strength = 0.2MG/4ML
----
Product id = 27894
Application Number = 9296
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPASIL-APRESOLINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG;0.2MG
----
Product id = 27893
Application Number = 9296
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPASIL-APRESOLINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 004
Tecode =
Rld = No
Strength = 25MG;0.1MG
----
Product id = 27357
Application Number = 9357
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RAU-SED
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27358
Application Number = 9357
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RAU-SED
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 004
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27359
Application Number = 9357
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RAU-SED
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 006
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 27360
Application Number = 9357
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RAU-SED
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 008
Tecode =
Rld = No
Strength = 1MG
----
Product id = 27829
Application Number = 9376
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SANDRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LILLY
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27828
Application Number = 9376
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SANDRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LILLY
ProductNo = 004
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27887
Application Number = 9391
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPANRAY
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PANRAY
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27888
Application Number = 9391
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPANRAY
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PANRAY
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27889
Application Number = 9391
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPANRAY
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PANRAY
ProductNo = 004
Tecode =
Rld = No
Strength = 1MG
----
Product id = 10773
Application Number = 9434
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPASIL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode =
Rld = No
Strength = 2.5MG/ML
----
Product id = 27897
Application Number = 9453
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPATE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = VALE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27898
Application Number = 9453
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPATE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = VALE
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27467
Application Number = 9627
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27468
Application Number = 9627
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = IMPAX LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 22156
Application Number = 9631
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HISERPIA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOWMAN PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 22157
Application Number = 9631
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HISERPIA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOWMAN PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27899
Application Number = 9645
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPIVITE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = VITARINE
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27451
Application Number = 9663
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BUNDY
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27452
Application Number = 9663
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BUNDY
ProductNo = 003
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27490
Application Number = 9667
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = VALEANT PHARM INTL
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27491
Application Number = 9667
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = VALEANT PHARM INTL
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27484
Application Number = 9838
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANDOZ
ProductNo = 001
Tecode = BP
Rld = No
Strength = 0.1MG
----
Product id = 27485
Application Number = 9838
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANDOZ
ProductNo = 002
Tecode = BP
Rld = Yes
Strength = 0.25MG
----
Product id = 27453
Application Number = 9859
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = CYCLE PHARMS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27454
Application Number = 9859
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = CYCLE PHARMS LTD
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 10736
Application Number = 10012
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SANDRIL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = LILLY
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG/ML
----
Product id = 27885
Application Number = 10124
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPALAN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LANNETT
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27886
Application Number = 10124
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPALAN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LANNETT
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27457
Application Number = 10441
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = EVERYLIFE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27458
Application Number = 10441
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = EVERYLIFE
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27459
Application Number = 10441
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = EVERYLIFE
ProductNo = 003
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 27460
Application Number = 10441
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = EVERYLIFE
ProductNo = 004
Tecode =
Rld = No
Strength = 1MG
----
Product id = 27469
Application Number = 11185
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27470
Application Number = 11185
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 20573
Application Number = 11635
Date of Application = 26,, Aug, 1987
RX/OTC/DISCN = DISCN
Tradename = DIUPRES-250
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 003
Tecode =
Rld = No
Strength = 250MG;0.125MG
----
Product id = 20574
Application Number = 11635
Date of Application = 26,, Aug, 1987
RX/OTC/DISCN = DISCN
Tradename = DIUPRES-500
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 006
Tecode =
Rld = No
Strength = 500MG;0.125MG
----
Product id = 27895
Application Number = 11878
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPASIL-ESIDRIX #1
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG;0.1MG
----
Product id = 27896
Application Number = 11878
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPASIL-ESIDRIX #2
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 005
Tecode =
Rld = No
Strength = 50MG;0.1MG
----
Product id = 22573
Application Number = 11958
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROPRES 25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 22574
Application Number = 11958
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROPRES 50
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 27871
Application Number = 12193
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SER-AP-ES
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 005
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 25314
Application Number = 12265
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NAQUIVAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SCHERING
ProductNo = 003
Tecode =
Rld = No
Strength = 0.1MG;4MG
----
Product id = 27822
Application Number = 12359
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SALUTENSIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SHIRE
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 27823
Application Number = 12359
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SALUTENSIN-DEMI
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SHIRE
ProductNo = 004
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 20577
Application Number = 12708
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DIUTENSEN-R
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MEDPOINTE PHARM HLC
ProductNo = 005
Tecode =
Rld = No
Strength = 2.5MG;0.1MG
----
Product id = 24388
Application Number = 12972
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = METATENSIN #2
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG;2MG
----
Product id = 24389
Application Number = 12972
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = METATENSIN #4
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 002
Tecode =
Rld = No
Strength = 0.1MG;4MG
----
Product id = 27413
Application Number = 13636
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RENESE-R
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG;0.25MG
----
Product id = 22572
Application Number = 13927
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROMOX R
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 27397
Application Number = 15103
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = REGROTON
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.25MG
----
Product id = 20168
Application Number = 15103
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DEMI-REGROTON
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 27493
Application Number = 80393
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27486
Application Number = 80446
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27462
Application Number = 80457
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27461
Application Number = 80457
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 002
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27463
Application Number = 80457
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 003
Tecode =
Rld = No
Strength = 1MG
----
Product id = 27471
Application Number = 80492
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MARSHALL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27472
Application Number = 80492
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MARSHALL PHARMA
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27474
Application Number = 80525
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MK LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27473
Application Number = 80525
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MK LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27480
Application Number = 80582
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PVT FORM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27482
Application Number = 80582
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PVT FORM
ProductNo = 002
Tecode =
Rld = No
Strength = 1MG
----
Product id = 27483
Application Number = 80637
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = REXALL
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27492
Application Number = 80679
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27448
Application Number = 80721
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27496
Application Number = 80723
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WHITEWORTH TOWN PLSN
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27497
Application Number = 80723
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WHITEWORTH TOWN PLSN
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27498
Application Number = 80723
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WHITEWORTH TOWN PLSN
ProductNo = 003
Tecode =
Rld = No
Strength = 1MG
----
Product id = 27495
Application Number = 80749
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 27478
Application Number = 80753
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27477
Application Number = 80753
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27464
Application Number = 80975
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27465
Application Number = 80975
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27466
Application Number = 80975
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 003
Tecode =
Rld = No
Strength = 1MG
----
Product id = 27449
Application Number = 83058
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BELL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27450
Application Number = 83058
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BELL PHARMA
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27455
Application Number = 83145
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ELKINS SINN
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 27456
Application Number = 83145
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ELKINS SINN
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 22380
Application Number = 83568
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.1MG
----
Product id = 22379
Application Number = 83571
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 22378
Application Number = 83572
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.1MG
----
Product id = 22381
Application Number = 83573
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22388
Application Number = 83666
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22391
Application Number = 83770
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE AND HYDRALAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 22575
Application Number = 83877
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROSERPINE PLUS (R-H-H)
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 22267
Application Number = 84291
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE HYDROCHLORIDE, HYDROCHLOROTHIAZIDE AND RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 22385
Application Number = 84466
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 22389
Application Number = 84467
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 27500
Application Number = 84579
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 27499
Application Number = 84580
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 22384
Application Number = 84603
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 20755
Application Number = 84617
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DRALSERP
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.1MG
----
Product id = 27476
Application Number = 84663
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PHARMAVITE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 22273
Application Number = 84714
Date of Application = 29,, Jun, 1982
RX/OTC/DISCN = DISCN
Tradename = HYDRO-RESERP
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABC HOLDING
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22275
Application Number = 84827
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRO-SERP "25"
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 19316
Application Number = 84853
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG;0.125MG
----
Product id = 22270
Application Number = 84876
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRAP-ES
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 18794
Application Number = 84897
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CAM-AP-ES
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TG UNITED LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 27475
Application Number = 84974
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 27487
Application Number = 85207
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TABLICAPS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 22276
Application Number = 85213
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRO-SERP "50"
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 29164
Application Number = 85248
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRICHLORMETHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG;4MG
----
Product id = 22386
Application Number = 85317
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 22049
Application Number = 85338
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = H.R.-50
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WHITEWORTH TOWN PLSN
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 27494
Application Number = 85401
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 22383
Application Number = 85420
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PHARMERAL
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22382
Application Number = 85421
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PHARMERAL
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 27507
Application Number = 85549
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE, HYDRALAZINE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 22269
Application Number = 85771
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE, HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 27481
Application Number = 85775
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PVT FORM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 29285
Application Number = 85893
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = UNIPRES
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 27479
Application Number = 86117
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PVT FORM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----
Product id = 29286
Application Number = 86298
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = UNIPRES
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 22387
Application Number = 86330
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 22390
Application Number = 86331
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22268
Application Number = 87085
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE HYDROCHLORIDE-HYDROCHLOROTHIAZIDE-RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 27870
Application Number = 87210
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SER-A-GEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 27508
Application Number = 87556
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE, HYDRALAZINE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 27509
Application Number = 87709
Date of Application = 13,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = RESERPINE, HYDROCHLOROTHIAZIDE, AND HYDRALAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 19318
Application Number = 87744
Date of Application = 6,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = CHLOROTHIAZIDE-RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG;0.125MG
----
Product id = 19319
Application Number = 87745
Date of Application = 6,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = CHLOROTHIAZIDE-RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG;0.125MG
----
Product id = 22558
Application Number = 88110
Date of Application = 22,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = HYDROFLUMETHIAZIDE AND RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22557
Application Number = 88127
Date of Application = 22,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = HYDROFLUMETHIAZIDE AND RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 19317
Application Number = 88151
Date of Application = 9,, Jun, 1983
RX/OTC/DISCN = DISCN
Tradename = CHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG;0.125MG
----
Product id = 27502
Application Number = 88189
Date of Application = 10,, May, 1984
RX/OTC/DISCN = DISCN
Tradename = RESERPINE AND HYDROCHLOROTHIAZIDE-50
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WEST WARD
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22556
Application Number = 88195
Date of Application = 26,, Oct, 1983
RX/OTC/DISCN = DISCN
Tradename = HYDROFLUMETHIAZIDE AND RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 27501
Application Number = 88200
Date of Application = 31,, Jan, 1984
RX/OTC/DISCN = DISCN
Tradename = RESERPINE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 19315
Application Number = 88365
Date of Application = 22,, Dec, 1983
RX/OTC/DISCN = DISCN
Tradename = CHLOROTHIAZIDE AND RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG;0.125MG
----
Product id = 27506
Application Number = 88376
Date of Application = 28,, Oct, 1983
RX/OTC/DISCN = DISCN
Tradename = RESERPINE, HYDRALAZINE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 19314
Application Number = 88557
Date of Application = 22,, Dec, 1983
RX/OTC/DISCN = DISCN
Tradename = CHLOROTHIAZIDE AND RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG;0.125MG
----
Product id = 27505
Application Number = 88570
Date of Application = 10,, Apr, 1984
RX/OTC/DISCN = DISCN
Tradename = RESERPINE, HYDRALAZINE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 27504
Application Number = 88907
Date of Application = 20,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = RESERPINE AND HYDROFLUMETHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 27503
Application Number = 88932
Date of Application = 11,, Jan, 1985
RX/OTC/DISCN = DISCN
Tradename = RESERPINE AND HYDROFLUMETHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 27489
Application Number = 89019
Date of Application = 7,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27488
Application Number = 89020
Date of Application = 7,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG
----