ropinirole-hydrochloride
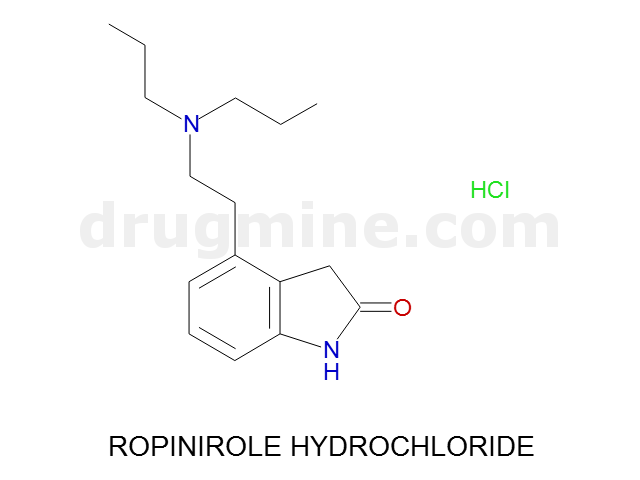


Name: ROPINIROLE HYDROCHLORIDE
ID :
MW: 260
Number of atoms: 19
Molecular_Formula: C16H24N2O
Alogp: 2.959
Indication class : Antiparkinsonian (D2 receptor agonist)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
ropinirole hydrochloride containing products summary
There are in total 126 different products containing the active ingredient ropinirole hydrochloride. From the 126 drug products, 8 have been discontinued.Product id = 27439
Application Number = 20658
Date of Application = 19,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = REQUIP
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 0.25MG BASE
----
Product id = 27440
Application Number = 20658
Date of Application = 19,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = REQUIP
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 27441
Application Number = 20658
Date of Application = 19,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = REQUIP
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 27442
Application Number = 20658
Date of Application = 19,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = REQUIP
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 27445
Application Number = 20658
Date of Application = 19,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = REQUIP
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27443
Application Number = 20658
Date of Application = 27,, Jan, 1999
RX/OTC/DISCN = RX
Tradename = REQUIP
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 3MG BASE
----
Product id = 27444
Application Number = 20658
Date of Application = 27,, Jan, 1999
RX/OTC/DISCN = RX
Tradename = REQUIP
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 007
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 16572
Application Number = 22008
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = REQUIP XL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 2MG BASE
----
Product id = 16573
Application Number = 22008
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = DISCN
Tradename = REQUIP XL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 3MG BASE
----
Product id = 16574
Application Number = 22008
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = REQUIP XL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 16576
Application Number = 22008
Date of Application = 13,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = REQUIP XL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 16577
Application Number = 22008
Date of Application = 31,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = REQUIP XL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 16575
Application Number = 22008
Date of Application = 10,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = REQUIP XL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 6MG BASE
----
Product id = 27777
Application Number = 77460
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.25MG BASE
----
Product id = 27778
Application Number = 77460
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 27779
Application Number = 77460
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 27780
Application Number = 77460
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 27781
Application Number = 77460
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 3MG BASE
----
Product id = 27782
Application Number = 77460
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 27783
Application Number = 77460
Date of Application = 19,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 007
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27770
Application Number = 77852
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.25MG BASE
----
Product id = 27771
Application Number = 77852
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 27772
Application Number = 77852
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 27773
Application Number = 77852
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 27774
Application Number = 77852
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 3MG BASE
----
Product id = 27775
Application Number = 77852
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 27776
Application Number = 77852
Date of Application = 19,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 007
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27763
Application Number = 78110
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.25MG BASE
----
Product id = 27764
Application Number = 78110
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 27765
Application Number = 78110
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 27766
Application Number = 78110
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 27767
Application Number = 78110
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 3MG BASE
----
Product id = 27768
Application Number = 78110
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 27769
Application Number = 78110
Date of Application = 11,, Jul, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 007
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27735
Application Number = 78230
Date of Application = 20,, May, 2008
RX/OTC/DISCN = DISCN
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.25MG BASE
----
Product id = 27736
Application Number = 78230
Date of Application = 20,, May, 2008
RX/OTC/DISCN = DISCN
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 27737
Application Number = 78230
Date of Application = 20,, May, 2008
RX/OTC/DISCN = DISCN
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 27738
Application Number = 78230
Date of Application = 20,, May, 2008
RX/OTC/DISCN = DISCN
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 27739
Application Number = 78230
Date of Application = 20,, May, 2008
RX/OTC/DISCN = DISCN
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 3MG BASE
----
Product id = 27740
Application Number = 78230
Date of Application = 20,, May, 2008
RX/OTC/DISCN = DISCN
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 006
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 27741
Application Number = 78230
Date of Application = 20,, May, 2008
RX/OTC/DISCN = DISCN
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 007
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27749
Application Number = 78881
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.25MG BASE
----
Product id = 27750
Application Number = 78881
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 27751
Application Number = 78881
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 27752
Application Number = 78881
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 27753
Application Number = 78881
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 3MG BASE
----
Product id = 27754
Application Number = 78881
Date of Application = 5,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 27755
Application Number = 78881
Date of Application = 19,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 007
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27784
Application Number = 79050
Date of Application = 29,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.25MG BASE
----
Product id = 27785
Application Number = 79050
Date of Application = 29,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 27786
Application Number = 79050
Date of Application = 29,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 27787
Application Number = 79050
Date of Application = 29,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 27788
Application Number = 79050
Date of Application = 29,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 3MG BASE
----
Product id = 27789
Application Number = 79050
Date of Application = 29,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 27790
Application Number = 79050
Date of Application = 29,, May, 2008
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 007
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27728
Application Number = 79165
Date of Application = 7,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.25MG BASE
----
Product id = 27729
Application Number = 79165
Date of Application = 7,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 27730
Application Number = 79165
Date of Application = 7,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 27731
Application Number = 79165
Date of Application = 7,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 27732
Application Number = 79165
Date of Application = 7,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 3MG BASE
----
Product id = 27733
Application Number = 79165
Date of Application = 7,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 27734
Application Number = 79165
Date of Application = 7,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 007
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27756
Application Number = 79229
Date of Application = 28,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ORCHID HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.25MG BASE
----
Product id = 27757
Application Number = 79229
Date of Application = 28,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ORCHID HLTHCARE
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 27758
Application Number = 79229
Date of Application = 28,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ORCHID HLTHCARE
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 27759
Application Number = 79229
Date of Application = 28,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ORCHID HLTHCARE
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 27760
Application Number = 79229
Date of Application = 28,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ORCHID HLTHCARE
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 3MG BASE
----
Product id = 27761
Application Number = 79229
Date of Application = 28,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ORCHID HLTHCARE
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 27762
Application Number = 79229
Date of Application = 28,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ORCHID HLTHCARE
ProductNo = 007
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27742
Application Number = 90135
Date of Application = 25,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.25MG BASE
----
Product id = 27743
Application Number = 90135
Date of Application = 25,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 27744
Application Number = 90135
Date of Application = 25,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 27745
Application Number = 90135
Date of Application = 25,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 27746
Application Number = 90135
Date of Application = 25,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 3MG BASE
----
Product id = 27747
Application Number = 90135
Date of Application = 25,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 27748
Application Number = 90135
Date of Application = 25,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 007
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27791
Application Number = 90411
Date of Application = 1,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.25MG BASE
----
Product id = 27792
Application Number = 90411
Date of Application = 1,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 27793
Application Number = 90411
Date of Application = 1,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 27794
Application Number = 90411
Date of Application = 1,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 27795
Application Number = 90411
Date of Application = 1,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 3MG BASE
----
Product id = 27796
Application Number = 90411
Date of Application = 1,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 27797
Application Number = 90411
Date of Application = 1,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 007
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27721
Application Number = 90429
Date of Application = 24,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.25MG BASE
----
Product id = 27722
Application Number = 90429
Date of Application = 24,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 27723
Application Number = 90429
Date of Application = 24,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 27724
Application Number = 90429
Date of Application = 24,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 27725
Application Number = 90429
Date of Application = 24,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 3MG BASE
----
Product id = 27726
Application Number = 90429
Date of Application = 24,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 27727
Application Number = 90429
Date of Application = 24,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 007
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 16580
Application Number = 90869
Date of Application = 17,, May, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 16581
Application Number = 90869
Date of Application = 17,, May, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 16582
Application Number = 90869
Date of Application = 17,, May, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 6MG BASE
----
Product id = 16583
Application Number = 90869
Date of Application = 17,, May, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 16584
Application Number = 90869
Date of Application = 17,, May, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 16611
Application Number = 91395
Date of Application = 27,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 16612
Application Number = 91395
Date of Application = 27,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 16613
Application Number = 91395
Date of Application = 27,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 6MG BASE
----
Product id = 16614
Application Number = 91395
Date of Application = 27,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 16615
Application Number = 91395
Date of Application = 27,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WOCKHARDT LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 16606
Application Number = 200431
Date of Application = 6,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 16607
Application Number = 200431
Date of Application = 6,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 16608
Application Number = 200431
Date of Application = 6,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 6MG BASE
----
Product id = 16609
Application Number = 200431
Date of Application = 6,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 16610
Application Number = 200431
Date of Application = 6,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 16595
Application Number = 200462
Date of Application = 15,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 16596
Application Number = 200462
Date of Application = 15,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 3MG BASE
----
Product id = 16597
Application Number = 200462
Date of Application = 15,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 16598
Application Number = 200462
Date of Application = 15,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 6MG BASE
----
Product id = 16599
Application Number = 200462
Date of Application = 15,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 16600
Application Number = 200462
Date of Application = 15,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 16601
Application Number = 201047
Date of Application = 6,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 16602
Application Number = 201047
Date of Application = 6,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 16603
Application Number = 201047
Date of Application = 6,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 6MG BASE
----
Product id = 16604
Application Number = 201047
Date of Application = 6,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 16605
Application Number = 201047
Date of Application = 6,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 16590
Application Number = 201576
Date of Application = 6,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 16591
Application Number = 201576
Date of Application = 6,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 16592
Application Number = 201576
Date of Application = 6,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 6MG BASE
----
Product id = 16593
Application Number = 201576
Date of Application = 6,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 16594
Application Number = 201576
Date of Application = 6,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----
Product id = 16585
Application Number = 202786
Date of Application = 22,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 16586
Application Number = 202786
Date of Application = 22,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 16587
Application Number = 202786
Date of Application = 22,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 6MG BASE
----
Product id = 16588
Application Number = 202786
Date of Application = 22,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 16589
Application Number = 202786
Date of Application = 22,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = ROPINIROLE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 12MG BASE
----